- Department of Neurosurgery, Kyushu Rosai Hospital, Kokura Minami-Ku, Kitakyushu, Japan
- Department of Neurosurgery, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan
- Department of Neurosurgery, Fukuoka Children's Hospital, Higashi-ku, Fukuoka, Japan
- Department of Cerebrovascular Disease, Kyushu Rosai Hospital, Kokura Minami-Ku, Kitakyushu, Japan
Correspondence Address:
Takafumi Shimogawa
Department of Neurosurgery, Kyushu Rosai Hospital, Kokura Minami-Ku, Kitakyushu, Japan
DOI:10.4103/sni.sni_82_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Takafumi Shimogawa, Takato Morioka, Tetsuro Sayama, Tomoaki Akiyama, Sei Haga, Toshiyuki Amano, Yoshihiko Furuta, Kei Murao, Shuji Arakawa, Iwao Takeshita. Impact of low coagulation factor XIII activity in patients with chronic subdural hematoma associated with cerebrospinal fluid hypovolemia: A retrospective study. 14-Aug-2017;8:192
How to cite this URL: Takafumi Shimogawa, Takato Morioka, Tetsuro Sayama, Tomoaki Akiyama, Sei Haga, Toshiyuki Amano, Yoshihiko Furuta, Kei Murao, Shuji Arakawa, Iwao Takeshita. Impact of low coagulation factor XIII activity in patients with chronic subdural hematoma associated with cerebrospinal fluid hypovolemia: A retrospective study. 14-Aug-2017;8:192. Available from: http://surgicalneurologyint.com/?post_type=surgicalint_articles&p=8555
Abstract
Background:Cerebrospinal fluid hypovolemia (CSFH) is sometimes associated with chronic subdural hematomas (CSHs). Affected patients often develop enlargement and recurrence of the CSH, even if appropriate treatments such as epidural blood patch (EBP) and/or burr-hole surgery for the CSH are performed. This situation may lead to subclinical coagulopathy, including low coagulation factor XIII (CFXIII) activity. We retrospectively analyzed whether CFXIII activity was involved in the development of CSHs and post-treatment exacerbation of CSHs in patients with CSFH.
Methods:We diagnosed CSFH by radioisotope (RI), magnetic resonance imaging (MRI) and computed tomography (CT) findings, and CSH by CT and/or MRI findings. The plasma CFXIII activity was assessed on admission. All patients with CSFH initially received conservative treatments. When these treatments were ineffective, the patients underwent EBP and/or CSH surgery according to previously reported therapeutic strategies.
Results:Among 206 patients with CSFH, 19 developed CSHs. Fourteen patients with a thin hematoma underwent EBP and three with a thick hematoma underwent CSH surgery immediately after EBP on the same day. We were unable to diagnose two patients with CSFH at the time of admission, and one of these two patients underwent repeated CSH surgery before obtaining the correct diagnosis. Seven patients (36.8%) developed CSH exacerbation after the treatment. The CFXIII activity was significantly lower in patients with than without a CSH (42.1% vs. 12.8%, respectively; P = 0.003). The CFXIII activity was significantly lower in patients with than without post-treatment CSH exacerbation (P = 0.046). All five patients with low CFXIII activity who developed CSH exacerbation received intravenous injection of CFXIII and had no recurrence of CSH after the additional treatment.
Conclusion:In patients with CSFH, low CFXIII activity is one of the risk factors for both the development of a CSH and the post-treatment exacerbation CSH.
Keywords: Cerebrospinal fluid hypovolemia, chronic subdural hematoma, coagulation factor, coagulation factor XIII, intractable chronic subdural hematoma
INTRODUCTION
It is well known that chronic subdural hematomas (CSHs) are associated with cerebrospinal fluid hypovolemia (CSFH).[
With respect to the therapeutic strategy for a CSFH-associated CSH, Takahashi et al.[
Subclinical coagulopathy should be suspected in patients with an intractable CSH.[
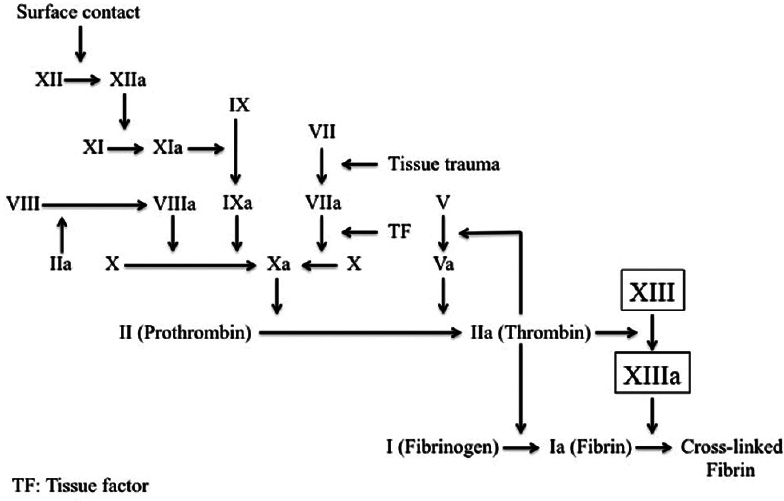
Figure 1
Coagulation cascade. The extrinsic and intrinsic pathways serve to activate coagulation factor (CF) X to Xa, a component of the prothrombinase complex that converts prothrombin (CFII) to thrombin (CFIIa). Thrombin activates CFXIII to XIIIa, which stabilizes the fibrin clot by covalently cross-linked fibrin. Abbreviations within figure: TF, tissue factor
Although, various CFs including CFVII, CFX, and CFXI could lead to intractable CSH,[
MATERIALS AND METHODS
Diagnosis of cerebrospinal fluid hypovolemia
Patients with orthostatic headache admitted to our department from 1994 to 2015, clinically suspected of having CSFH underwent radioisotope (RI) cisternography, brain magnetic resonance imaging (MRI), and/or computed tomography (CT). RI cisternography was performed to check for direct and indirect findings of CSF leakage. Direct findings were defined as focal areas of increased activity in unilateral or bilateral regions of the paraspinal area, and indirect findings were defined as early visualization of bladder activity (radioactivity in the urinary bladder 1–3 h after injection), no visualization of activity over the brain convexities (no remarkable accumulation around the brain convexities 24 h after injection), rapid disappearance of spinal activity (1–5 h after injection), and abnormal visualization of the root sleeves (asymmetric activity outlining the spinal nerve roots at any time after injection).[
MRI and/or CT were performed on all patients to exclude the presence of intracranial lesions such as tumors or hemorrhage. MRI with gadolinium enhancement was performed to check for diffuse meningeal enhancement. Analysis of brain descent was based on the incisural line and foramen magnum line on mid-sagittal MRI.
We diagnosed CSFH by RI, MRI, and CT findings. Evaluation of the radiological images was based on visual inspection by experienced neurosurgeons (I.T. and T.S.) and two radiologists. No differences in their interpretations were noted on independent assessments.
Diagnosis of chronic subdural hematoma
We diagnosed CSH by CT and/or MRI findings. The hematoma was defined as thin when its maximum thickness was <14 mm and as thick when its thickness was >15 mm. Patients with subdural hygromas were excluded from this study. Evaluation of CT and MRI findings was also based on visual inspection by experienced neurosurgeons (I.T. and T.S.) and radiologists. No differences in their interpretations were noted on independent assessments.
Clinical assessment for coagulation factor XIII activity and other factors
We assessed the CFXIII activity (normal range, 70–140%) in plasma obtained on admission in 206 patients. From 1994 to 2006 (Cases 5, 7, 12, 15, 17 and 19), we recorded whether the CFXIII activity was <70% or ≥70%; from 2007 onward, we recorded the detailed CFXIII activity. CFXIII activity of ≥70% was defined as normal.
Furthermore, factors possibly related to post-treatment CSH exacerbation were statistically analyzed. Patient variables included sex, age, CSH site, hematoma thickness at first treatment, direct findings, indirect findings, and MRI findings (diffuse meningeal enhancement and descent of brain).
Therapeutic strategy
All patients with CSFH initially received conservative treatments such as bed rest, analgesics, generous oral fluid intake, and fluid drip infusion. When these treatments were ineffective as evidenced by clinical and/or neuroradiological findings, the patients underwent EBP and/or CSH surgery according to the similar strategies reported by Takahashi et al.[
The performance of EBP involved injection of autologous blood into the epidural space at the level of the cervical, thoracic, or lumbar vertebra according to the RI cisternography results. For CSH surgery, patients underwent a standard neurosurgical procedure with full evacuation of all hematoma compartments including several washing steps with saline solution through a single burr-hole. A temporary soft silicone subdural drain was placed in the subdural space and connected to a closed drainage system for 12 to 24 h. Patients with bilateral CSHs received the same treatment on both sides. EBP and CSH surgery were performed with the same techniques by experienced neurosurgeons.
Diagnosis of chronic subdural hematoma exacerbation and additional therapeutic strategy
We diagnosed patients with CSH enlargement or recurrence (CSH exacerbation group) by evaluating repeated CT scans after the treatment. We performed repeated CT scans on postoperative day 1, day 7, and day 14 routinely. CSH enlargement was diagnosed when the maximum post-treatment hematoma thickness was larger than the pretreatment thickness. We performed reoperation when the hematoma became large in thickness.
In the CSH exacerbation group, a purified pasteurized CFXIII concentrate (Fibrogammin P; CSL Behring, Tokyo, Japan) was intravenously administered at 24 mL/day for 5 days if the CFXIII activity was <70%.
All patients were followed up to check whether there were serious complications and recurrence of CSH or not after the last treatment (follow-up period; mean: 8.0 years, range: 2–21 years).
Statistical analysis
JMP 11.0 software (SAS Institute, Cary, NC, USA) was used to perform the statistical analyses. Univariate analyses were conducted to determine the relationship between the development of CSFH-associated CSH and low CFXIII activity, and the risk factors for CSFH-associated CSH exacerbation after EBP and/or CSH surgery. We compared CSH exacerbation and non-exacerbation using t-statistics for continuous variables and χ2 statistics for categorical variables. The risk factors for CSFH-associated CSH exacerbation after EBP and CSH surgery were analyzed with only univariate analysis because of the small sample size. A P value of <0.050 was considered statistically significant.
The clinical data, treatments, and outcomes were obtained from the medical records. All patients provided written informed consent.
RESULTS
In total, 206 patients were conclusively diagnosed with CSFH based on RI, MRI, and CT findings. The patients comprised 95 males and 111 females aged 12 to 72 years (mean, 36.2 years).
Characteristics of patients with CSFH-associated CSH
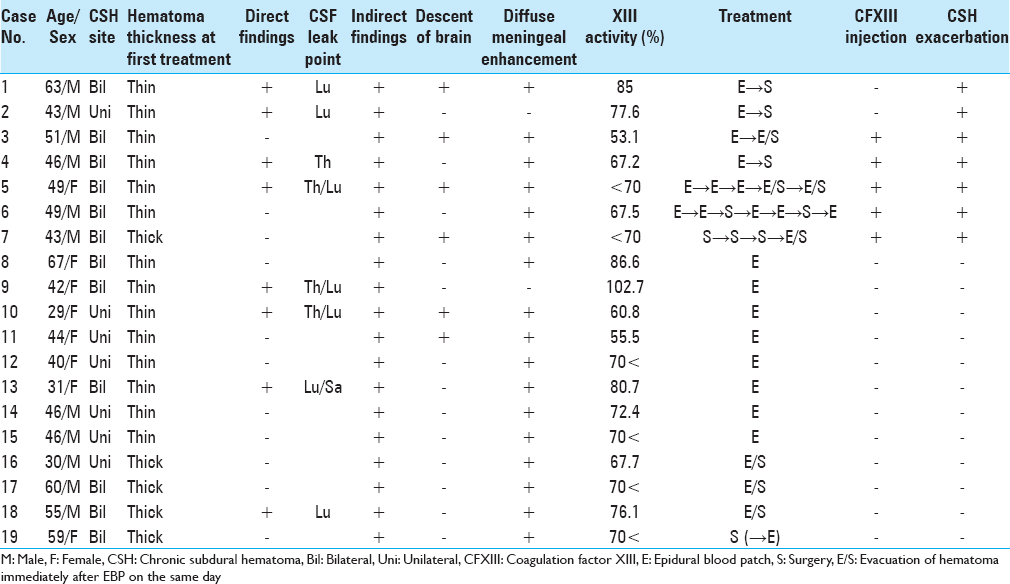
Nineteen of 206 patients (9.2%) had a CSFH-associated CSH (CSH occurrence group) [
Characteristics of patients with chronic subdural hematoma exacerbation after the first treatment
Seven patients (36.8%) developed CSH exacerbation after the first treatment (CSH exacerbation group). Six patients underwent EBP first followed by an additional EBP and/or CSH surgery 1 or 2 days later. One patient (Case 7) underwent repeated CSH surgery before the correct diagnosis was obtained and an additional CSH surgery immediately after EBP on the same day after the diagnosis was obtained. Twelve patients did not develop CSH exacerbation after the first treatment (non-CSH exacerbation group) [
Relationship between development of CSFH-associated CSH and low CFXIII activity
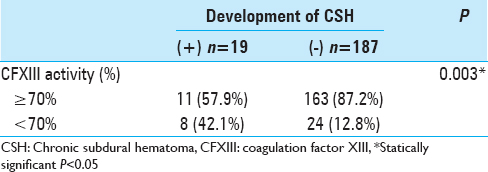
In a comparison of the CSH occurrence group and non-CSH occurrence group, a univariate analysis was performed to evaluate the relationship between the development of CSFH-associated CSH and low CFXIII activity. Low CFXIII activity was significantly associated with CSH occurrence (42.1% vs. 12.8%, P = 0.003) [
Relationship between post-treatment CSH exacerbation and low CFXIII activity
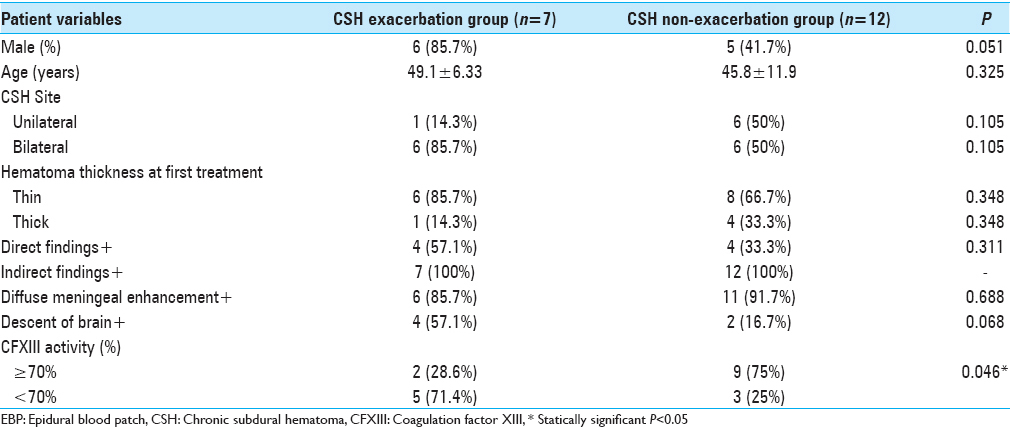
In the CSH exacerbation group, five patients (71.4%) (Cases 3, 4, 5, 6, and 7) had low CFXIII activity and two (28.6%) had normal CFXIII activity. In the non-CSH exacerbation group; however, nine patients (75.0%) had normal CFXIII activity and three (25.0%) had low CFXIII activity. Even among patients with low CFXIII activity, three patients (Cases 10, 11, and 16) had no recurrence of CSH after the first treatment. In particular, Case 16 had a thick hematoma and low CFXIII activity, but did not develop exacerbation of the hematoma following CSH surgery immediately after EBP on the same day. However, the univariate analyses revealed that low CFXIII activity was significantly associated with CSH exacerbation. Other factors were not statistically significant [
In the CSH exacerbation group, all five patients with low CFXIII activity (Cases 3, 4, 5, 6, and 7) received intravenous injection of CFXIII. Three patients experienced no recurrence of CSH just after the second treatment (Cases 3, 4, and 7), and two patients finally had no recurrence of CSH after repeated XIII injections and additional treatments (Cases 5 and 6).
Representative case (Case 3)
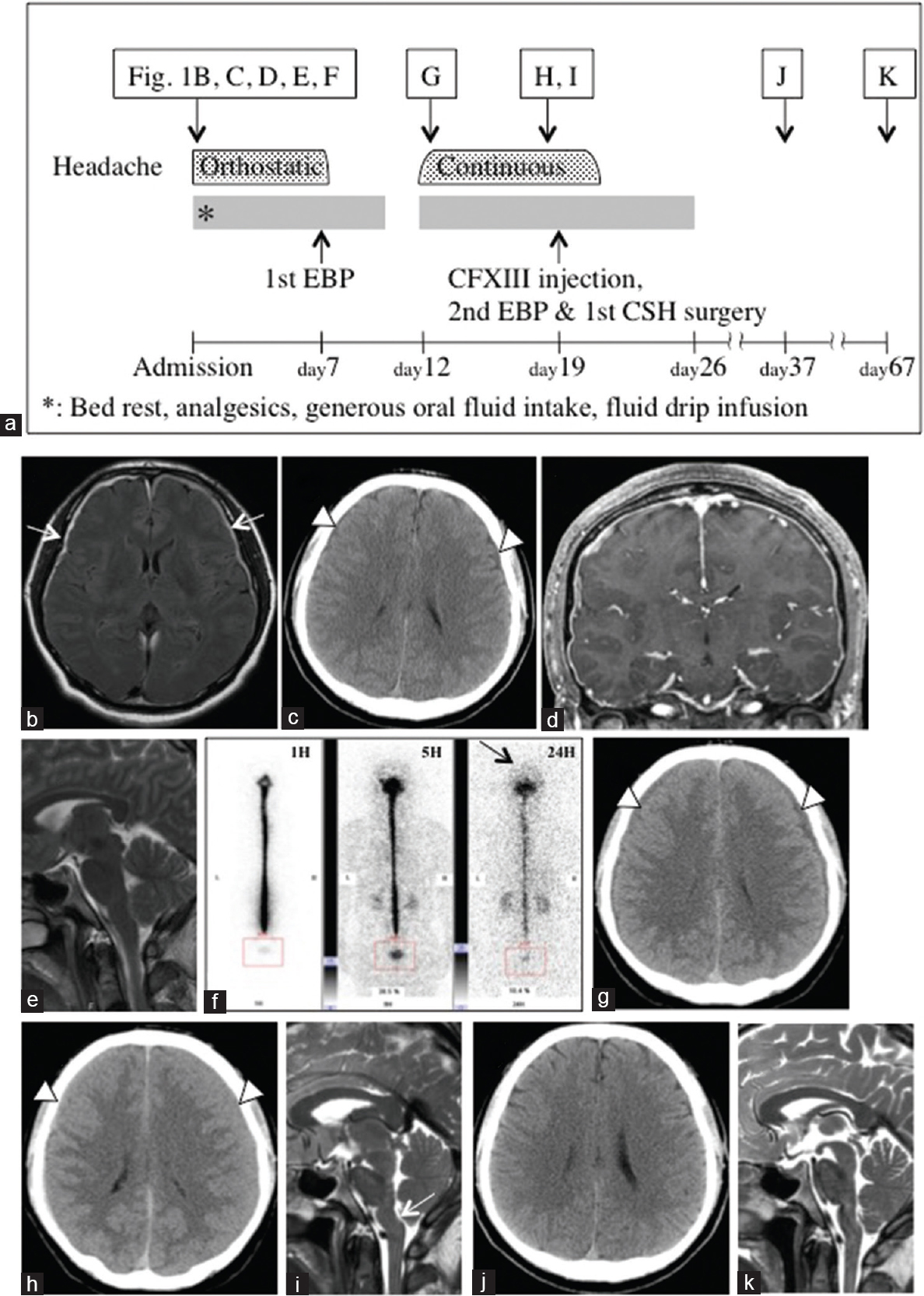
Case 3 is a representative case, and his clinical course is shown in
Figure 2
(a) Clinical course. (b) MRI with FLAIR on admission showed thin chronic subdural hematomas (CSHs) on both sides (white arrows). (c) Axial CT depicted bilateral thin CSHs. (d) Coronal T1-weighted MRI with gadolinium showed diffuse meningeal enhancement. (e) No displacement of the brain stem was seen on the mid-sagittal T2-weighted MRI. (f) Although RI cisternography 1, 5, and 24 h after intrathecal injection of RI revealed no apparent cerebrospinal fluid leakage, no visualization of RI activity over the cerebral convexities was seen even after 24 h. (g) Axial CT scan on the 5th day after EBP demonstrated that the CSHs were slightly increased in size. (h) Axial CT scan on the 12th day after the first EBP depicted that the thin CSHs had apparently increased. (i) Mid-sagittal T2-weighted MRI on the 12th day after the first EBP showed backward displacement of the brain stem. (j) Axial CT scan on the 18th day after the second treatment revealed complete disappearance of the CSHs. (k) Disappearance of the brain stem displacement was seen on mid-sagittal T2-weighted MRI on the 48th day after the second treatment. Abbreviations within figure: EBP, epidural blood patch; CFXIII, coagulation factor XIII; CSH, chronic subdural hematoma
DISCUSSION
The present study revealed that low CFXIII activity was significantly associated with CSH occurrence vs. non-occurrence (42.1% vs. 12.8%, respectively; P = 0.003). Bosche B et al.[
To the best of our knowledge, a systematic study of the risk factors for CSFH-associated CSH exacerbation after treatment has not been performed. Previous authors[
Five of our patients with low CFXIII activity developed CSH exacerbation after treatment. All patients received CFXIII injection and had no recurrence of CSH. From the perspective of CSFH treatment, intravenous administration of CFXIII accelerates healing of CSF leak sites.[
There are several limitations of the present study. First, the sample size was relatively small. Second, a variety of pathophysiologies associated with CSFH might affect the exacerbation of the hematoma. Despite low CFXIII activity, three patients (Cases 10, 11, and 16) had no recurrence of CSH after the first treatment. In particular, Case 16 (thick hematoma and low CFXIII activity) did not develop exacerbation of the hematoma following CSH surgery immediately after EBP on the same day. Third, we only focused on CFXIII and other standard coagulant parameters were not considered. Fourth, whether CSFH patients without CSH underwent anticoagulant agents and had platelet dysfunction and liver disease or not were not examined. Therefore, further studies with larger cohorts should be conducted.
CONCLUSIONS
This preliminary study indicates that low CFXIII activity is one of the risk factors for the development of CSHs and exacerbation of CSHs after treatment in patients with CSFH. Intravenous injection of CFXIII may help to prevent CSH exacerbation. Thus, measurement of CFXIII activity could be considered in the management of intractable CSHs associated with CSFH.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Achneck HE, Sileshi B, Parikh A, Milano CA, Welsby IJ, Lawson JH. Pathophysiology of bleeding and clotting in the cardiac surgery patient: From vascular endothelium to circulatory assist device surface. Circulation. 2010. 122: 2068-77
2. Albanese A, Tuttolomondo A, Anile C, Sabatino G, Pompucci A, Pinto A. Spontaneous chronic subdural hematomas in young adults with a deficiency in coagulation factor XIII. Report of three cases. J Neurosurg. 2005. 102: 1130-2
3. Barton JC, Bertoli LF, O’Malley S. Bilateral subdural hematomas in an adult with hereditary factor VII deficiency: A complication of sit-ups and inversion?. Clin Appl Thromb Hemost. 2009. 15: 242-4
4. Beck J, Gralla J, Fung C, Ulrich CT, Schucht P, Fichtner J. Spinal cerebrospinal fluid leak as the cause of chronic subdural hematomas in nongeriatric patients. J Neurosurg. 2014. 121: 1380-7
5. Bosche B, Molcanyi M, Noll T, Kochanek M, Kraus B, Rieger B. Occurrence and recurrence of spontaneous chronic subdural haematoma is associated with a factor XIII deficiency. Clin Neurol Neurosurg. 2013. 115: 13-8
6. Chung SJ, Lee JH, Kim SJ, Kwun BD, Lee MC. Subdural hematoma in spontaneous CSF hypovolemia. Neurology. 2006. 67: 1088-9
7. Dardik R, Loscalzo J, Inbal A. Factor XIII (FXIII) and angiogenesis. J Thromb Haemost. 2006. 4: 19-25
8. Dufner GS, Marbet GA. Factor XIII in man: A review. Hamostaseologie. 2002. 22: 11-9
9. Edahiro Y, Ichikawa K, Suzuki H, Yasuda H, Koike M, Komatsu N. Successful perioperative management of factor XI deficiency with administration of fresh-frozen plasma in a subdural hematoma patient. Geriatr Gerontol Int. 2016. 16: 143-4
10. Fadoo Z, Merchant Q, Rehman KA. New developments in the management of congenital Factor XIII deficiency. J Blood Med. 2013. 28: 4:65-73
11. Jansen JW, Haverkate F, Koopman J, Nieuwenhuis HK, Kluft C, Boschman TA. Influence of factor XIIIa activity on human whole blood clot lysis in vitro . Thromb Haemost. 1987. 7: 171-5
12. Morioka T, Aoki T, Tomoda Y, Takahashi H, Kakeda S, Takeshita I. Cerebrospinal fluid leakage in intracranial hypotension syndrome: Usefulness of indirect findings in radionuclide cisternography for detection and treatment monitoring. Clinical Nuclear Medicine. 2008. 33: 181-5
13. Muszbek L, Bagoly Z, Bereczky Z, Katona É. The involvement of blood coagulation factor XIII in fibrinolysis and thrombosis. Cardiovasc Hematol Agents Med Chem. 2008. 6: 190-205
14. Nagatani K, Takeuchi S, Wada K, Mori K, Shima K. Treatment of spontaneous intracranial hypotension with intravenous Factor XIII administration: Initial clinical experience. Turk Neurosurg. 2015. 25: 69-72
15. Nakajima H, Sakai T, Aoki N, Takakura K. Bilateral chronic subdural hematomas associated with intracranial hypotension. Neurol Med Chir. 1996. 36: 647-9
16. Noll T, Wozniak G, McCarson K, Hajimohammad A, Metzner HJ, Inserte J. Effect of factor XIII on endothelial barrier function. J Exp Med. 1999. 189: 1373-82
17. Schievink WI, Maya MM, Moser FG, Tourje J. Spectrum of subdural fluid collection in spontaneous intracranial hypotension. J Neurosurg. 2005. 103: 608-13
18. Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006. 295: 2286-96
19. Schievink WI, Maya MM, Pikul BK, Louy C. Spontaneous spinal cerebrospinal fluid leaks as the cause of subdural hematomas in elderly patients on anticoagulation. J Neurosurg. 2010. 112: 295-9
20. Senturk S, Guzel E, Bayrak AH, Bukte Y, Guzel A. Factor X deficiency presenting with bilateral chronic subdural hematoma. Pediatr Neurosurg. 2016. 46: 54-7
21. Takahashi K, Mima T, Akiba Y. Chronic subdural hematoma associated with spontaneous intracranial hypotension: Therapeutic strategies and outcomes of 55 cases. Neurol Med Chir. 2016. 15: 69-76
22. Ternström L, Radulovic V, Karlsson M, Baghaei F, Hyllner M, Bylock A. Plasma activity of individual coagulation factors, hemodilution and blood loss after cardiac surgery: A prospective observational study. Thromb Res. 2010. 126: e128-33
23. Yamashima T, Yamamoto S, Friede RL. The role of endothelial gap junctions in the enlargement of chronic subdural hematomas. J Neurosurg. 1983. 59: 298-303