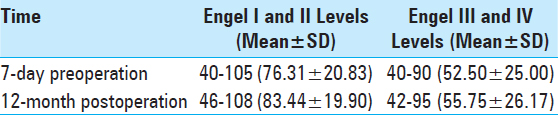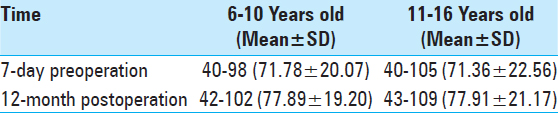- Department of Neurosurgery, Auhui Province, Anhui Provincial Hospital Affiliated to Anhui Medical University, Hefei, China
- Anhui Medical University, 81 Meishang Road, Hefei, China
- Department of Paediatrics, The First People's Hospital of Hefei, Huaihe Road, Hefei, China
Correspondence Address:
Xiangping Wei
Department of Neurosurgery, Auhui Province, Anhui Provincial Hospital Affiliated to Anhui Medical University, Hefei, China
DOI:10.4103/sni.sni_381_17
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Yang Wang, Dasheng Wang, Dingyi Li, Ruobing Qian, Xianming Fu, Chaoshi Niu, Jinghua Huang, Xiaohong Wen, Xiangping Wei. Improvement of intellectual outcomes in 20 children with refractory epilepsy after individualized surgery. 08-Oct-2018;9:203
How to cite this URL: Yang Wang, Dasheng Wang, Dingyi Li, Ruobing Qian, Xianming Fu, Chaoshi Niu, Jinghua Huang, Xiaohong Wen, Xiangping Wei. Improvement of intellectual outcomes in 20 children with refractory epilepsy after individualized surgery. 08-Oct-2018;9:203. Available from: http://surgicalneurologyint.com/surgicalint-articles/9027/
Abstract
Background:Refractory epilepsy is a common and troublesome neurosurgical disease. This study is designed to compare seizure control and degrees in intellectual outcome in children with refractory epilepsy after surgical treatment.
Methods:20 children with refractory epilepsy were treated with tailored epilepsy surgery or vagus nerve stimulation (VNS). We used the Engel Epilepsy Surgery Outcome Scale to evaluate seizure control and the Wechsler Intelligence Scale for Children, fourth edition (WISC-IV), to test the children's intellectual outcomes 7-day preoperative and 3-, 6-, and 12-month postoperative.
Results:In total, 14 cases were seizure free (Engel I) and 2 cases to have suffered few attacks since surgery (Engel II). In two cases, the frequency of seizures decreased by >90% (Engel III). In the remaining two cases, the effects of surgery on seizure control were not obvious (Engel IV). All children completed the WISC-IV test. On average, postoperative intelligence quotient (IQ) increased by 6.35 points 12-month postsurgery compared with the results of the preoperative tests (P 3.88 points compared with in the Engel III and IV groups (P
Conclusion:Individualized epilepsy surgery is safe and effective for children with refractory epilepsy. It can control or reduce the frequency of postoperative attacks as well as improve postoperative intellectual outcomes to different degrees.
Keywords: Engel epilepsy surgery outcome scale, individualized surgery, intellectual outcome, refractory epilepsy children, Wechsler intelligence scale for children fourth edition
INTRODUCTION
The prevalence of epilepsy in China may be as high as 7%. There are about 10 millions of people with epilepsy, 5% of whom have active epilepsy; for about 60% of epileptic patients, symptoms begin to appear in childhood. Almost two-thirds of secondary-epilepsy cases gradually evolve into intractable epilepsy.[
The purpose of this study is to compare presurgical with postsurgical cognitive function in children with epilepsy who underwent individualized surgery by using the Wechsler Intelligence Scale for Children, fourth edition (WISC-IV). Simultaneously, by using the Engel Epilepsy Surgery Outcome Scale, we investigated the effect of epilepsy surgery on postoperative intellectual-outcome improvement.[
METHODS
Study population
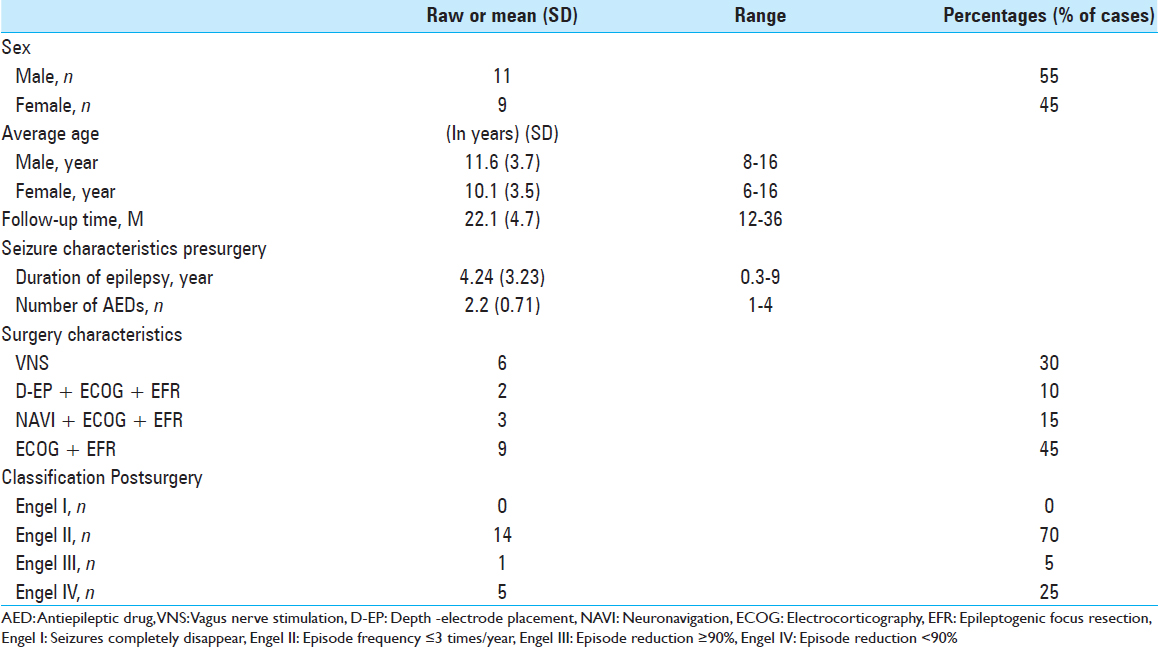
We analyzed 20 pediatric patients with intractable epilepsy who were admitted to the Department of Neurosurgery, Provincial Hospital Affiliated, Anhui Medical University, Hefei, China, from January 2014 to December 2016 retrospectively. In total, 11 cases were male and 9 cases were female. Age range was 6–16 years old (mean 11 ± 3.3-year old). Disease course was 3 months to 9 years, and average duration was 5.3 years [
Multimodal imaging examination
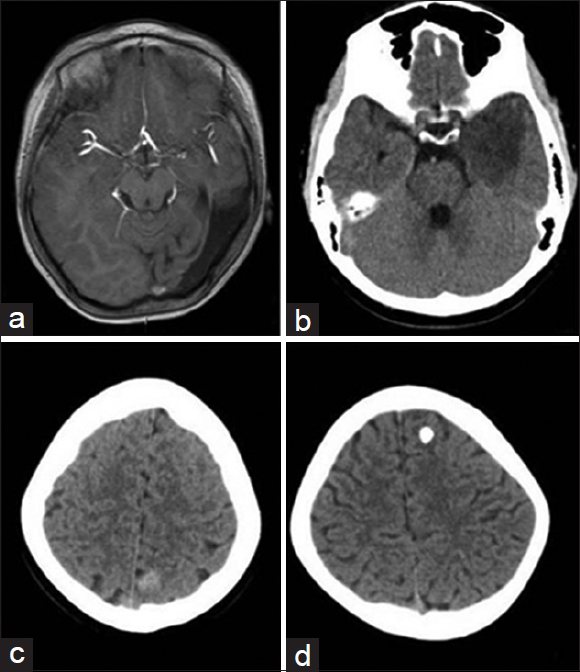
We examined the 20 children by multimodal imaging before surgery, using normal head computerized tomography (CT) scan, head magnetic resonance imaging (MRI) scan and enhancement, MRI fluid-attenuated inversion recovery sequence, MR spectroscopy imaging, and diffusion-weighted imaging. Results suggested that abnormal lesions of the frontal lobe occurred in two cases, which tended to be space-occupying cysts and astrocytoma. Abnormal lesions of the temporal and occipital lobes occurred in 10 cases, which tended to be space-occupying cysts, cortical dysplasia, cavernous hemangioma, and neuroepithelial tumors. No obvious abnormalities appeared in four cases. Abnormal signaling in the peripheral region of the lateral ventricle occurred in one case. Abnormal signaling in both hippocampi occurred in two cases, which tended to be hippocampal sclerosis. Cerebral developmental deformity occurred in one case [
Figure 1
Multimodal Imaging Examination. (a) Local low signaling in left temporal and occipital lobes hint at dysplasia. (b) Low-density shadow is indicated for low-grade glioma of temporal lobe. (c) Identical density focus shows parietal cavernous hemangioma. (d) High density shows frontal calcification focus
Electrophysiological examination
All 20 patients, once admitted to the hospital, received long-term video electroencephalography (VEEG) monitoring of the scalp in the epilepsy surgical ward. Monitoring time was 24–98 hours (average time, 50.3 ± 19 hours). Abnormal discharge was recorded clearly for all the patients, and epileptogenic focus was determined elementary combined with imaging examination. We could not locate specific epilepsy areas in two cases, since the monitoring results of the long-term scalp VEEG mainly showed diffuse slow waves extended throughout the bilateral frontotemporal lobe, so we decided to conduct depth-electrode placement to fully understand the relationship of time and space of epileptic seizures according to the deep-discharge sequences of the electrodes. Similarly, a typical attack was also recorded at least; simultaneously, intraoperative electrocorticogram (ECOG) cleared and defined epileptogenic focus any further and determined if the tumor is resected totally.[
Intellectual-outcome assessment
This study used the Chinese edition of WISC-IV adjusted to 6- to 16-year-old to evaluate intellectual outcomes in all 20 cases. Scale classifications of intelligence were high (110 points), medium (90–109 points), average (80–89 points), borderline (70–79 points), and extremely low (<69 points).[
Surgical procedures
Overall, eleven cases accepted intraoperative ECOG and epileptic-focus resection, six cases accepted vagus nerve stimulation (VNS), one case accepted parietal-lobe cavernous hemangioma resection under neuronavigation, one case accepted ependymal-tumor resection by subfrontal approach, and the final case accepted ECOG and anterior temporal-lobe and hippocampus resection [
Statistical methods
We used SPSS version 19.0 statistical software for statistical processing. Measurement of data were expressed with x ± SD. The t-test was used to compare preoperative and postoperative data. We set the standard inspection level at α = 0.05, and P < 0.05 was regarded as statistically significant.
RESULTS
Seizure control
We recorded seizure-control status in all 20 cases through outpatient review or telephone follow-up for 12–36 months (average, 22 months). Results based on Engel classification are shown in
Postoperative pathology
Fourteen children underwent surgery, and postoperative tissue specimens received pathological diagnoses. Results were as follows: four cases had focal cortical dysplasia with histopathological involvement of glial-cell vacuolar hyperplasia, layer pyramidal neuron proliferation, inflammatory-cell infiltration, lymphocytes, and foam cell infiltration. Three cases had dysembryoplastic neuroepithelial tumors, two cases had cortical dysplasia with microglial-nodule formation, one case had pilocytic astrocytoma (WHO I), one case had cavernous hemangioma with surrounding glial-cell hyperplasia, one case had ependymoma, one case had hippocampal sclerosis, and one case had mixed glial–neuronal tumors.
Intellectual-outcome assessment
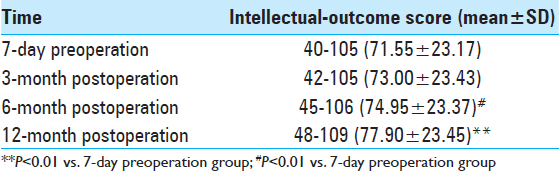
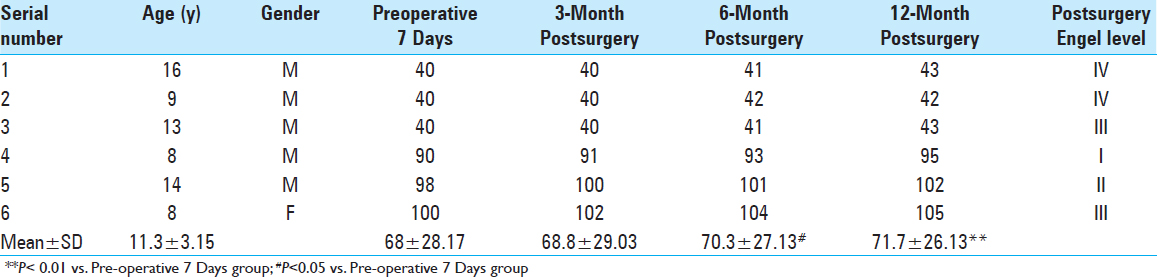
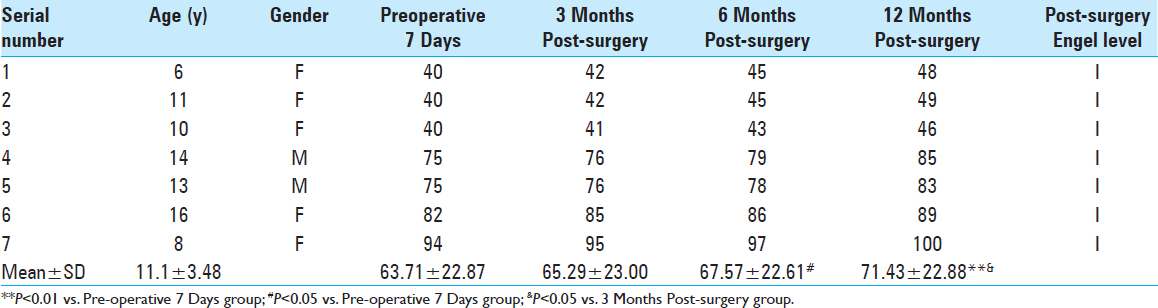
Intellectual-outcome variation before and after surgery. We assessed all 20 cases using WISC-IV before and after surgery. Postoperative intelligence quotient (IQ) scores increased by various degrees during different postoperative periods. Twelve months postoperation, IQ increased by 6.35 points compared with the results of the preoperative test (P < 0.01). Intellectual-outcome improvement was statistically significant following surgical intervention [
Surgical procedures affected post-operative intellectual outcomes. In the 6 cases that underwent VNS, cognitive function 12 months after surgery increased by 3.70 points compared with the results of the preoperative test, and the improvement was statistically significant (P < 0.01;
Effects of postoperativ Engel grading on intellectual outcome. The results showed that intellectual outcomes in the Engel I and II groups increased by 3.88 points more than in the Engel III and IV groups (P < 0.05). Engel grading had a positive effect on the prognosis by improving intelligence. This effect could be time dependent; recurrent attacks of epilepsy influenced brain development [
Effects of age group on intellectual outcome. Results showed that intellectual outcomes in the age group of 6–10 years decreased by 0.44 points compared with those in the age group of 11–16 years (P > 0.05). Time of surgery did not result in obvious differences in prognosis for improving intelligence between the two age groups [
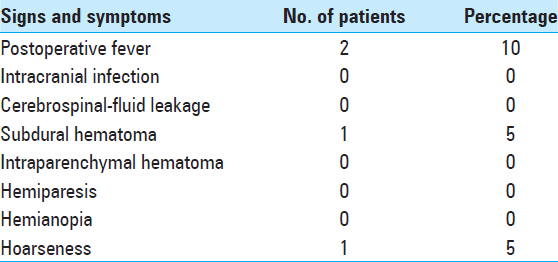
Postoperative complications and adverse effect. All operations were completed successfully. Postoperative head CT scan revealed that secondary bleeding, postoperative infection, cerebrospinal-fluid (CSF) leakage and other severe complications did not occur. Postoperative fever occurred in two cases, in which intracranial infection was eliminated as a cause via CSF examination. However, postoperative subcutaneous hematoma occurred in one case; the hematoma was reabsorbed after 1 week of treatment with a pressure bandage. In one case, hoarseness occurred temporarily after VNS and vanished four days later. There was no death over the long-term follow-up. Long-term hemiplegia, aphasia, or other complications did not appear [
DISCUSSION
The International League Against Epilepsy defines refractory epilepsy as that for which symptoms are not controlled by a combination of two standard antiepileptic drugs (AEDs).[
Age is frequently regarded as an extremely important determinant of preoperative evaluation, surgical occasion, surgical indications, and choice of individualized surgical procedure. The rapid development and maturation of the CNS in young children is a major cause of the manifestation of clinical complications.[
Previous studies have shown that surgery for temporal-lobe epilepsy (TLE) performed in childhood results in excellent long-term seizure control and favorable cognitive outcomes along with positive effects on brain development.[
The average IQ of the 20 children was 40–105 (71.55 ± 23.17) 7 days before surgery and reached 48–109 (77.90 ± 23.45) 12 months after surgery; their intellectual ability increased by 6.35 points above their highest previous scores (P < 0.01). Average IQ was closely associated with postoperative recovery time and tended to increase over time based on comparison of multiple time points Intellectual outcomes in the six cases treated with VNS increased by 3.70 points 12 months after surgery compared with the results of the preoperative test (P < 0.01). In the seven cases that underwent temporal-lobe lesion resection, it increased by 7.72 points (P < 0.01) 12-month postoperation. Two kinds of different surgical options both have positive effects on intellectual outcomes Intellectual outcomes in children in the Engel I and II groups increased by 3.88 points post-surgery compared with those in the Engel III and IV groups (P < 0.05). Engel grading had a positive effect on prognosis by improving intelligence, which further corroborates the finding that recurrent attacks of epilepsy influence brain development Postoperative intellectual outcomes in children in the age group of 6–10 years decreased by 0.44 points, which was more than in the age group of 11–16 years (P > 0.05). Surprisingly, the choice of surgery did not result in obvious differences in prognosis for improving intelligence between the different age groups.
The anterior temporal lobe and hippocampus play important roles in learning, memory, and other cognitive functions. If the bilateral anterior temporal lobe and hippocampus are damaged, patients may suffer severe cognitive impairments, including impairments to learning and memory.[
Preoperative detailed assessment and focal epileptogenic localization are key factors in the reduction of complications Epilepsy control in children reduces secondary impairments caused by abnormal discharge of brain tissue The reduction of the AED can decrease medication side effects include central nervous system damage The better physical and psychological status of children permits them to better integrate into society and a more enjoyable environment to improve their cognitive function further.
There are some limitations to our research. WISC-IV is a test designed to measure intelligence, rather than the other cognitive domains. It is insufficient for measuring types of cognitive problems beyond its defined testing range, such as those of memory, continuous attention, executive function, and language, which are often reported in epileptic children.
CONCLUSIONS
Early and individualized surgical intervention is an effective method for treatment of refractory epilepsy in children, and the effects of surgery on intellectual outcomes should be given greater attention. The positive effects of surgery are of great significance to improving intellectual outcomes for epileptic children, which are namely to minimize seizures and reduce adverse effects caused by long-term medication.
Financial support and sponsorship
This study was supported by grants from the Anhui Provincial Natural Science Foundation of China (No. 1508085QH184) and Hefei city independent innovation policy fund project of borrowing and replacing (No. YW201512010001).
Conflicts of interest
There are no conflicts of interest.
References
1. Amlerova J, Cavanna AE, Bradac O, Javurkova A, Raudenska J, Marusic P. Emotion recognition and social cognition in temporal lobe epilepsy and the effect of epilepsy surgery. Epilepsy Behav. 2014. 36: 86-9
2. Boly M, Jones B, Findlay G, Plumley E, Mensen A, Hermann B. Altered sleep homeostasis correlates with cognitive impairment in patients with focal epilepsy. Brain. 2017. 140: 1026-40
3. Carreño M, Donaire A, Sánchez-Carpintero R. Cognitive disorders associated with epilepsy: Diagnosis and treatment. Neurologist. 2008. 14: S26-34
4. Edelvik A, Taft C, Ekstedt G, Malmgren K. Health-related quality of life and emotional well-being after epilepsy surgery: A prospective, controlled, long-term follow-up. Epilepsia. 2017. 58: 1706-15
5. Engineer CT, Hays SA, Kilgard MP. Vagus nerve stimulation as a potential adjuvant to behavioral therapy for autism and other neurodevelopmental disorders. J Neurodev Disord. 2017. 9: 20-
6. Gallagher A, Jambaqué I, Lassonde M. Cognitive outcome of surgery. Handb Clin Neurol. 2013. 111: 797-802
7. Gulati S, Yoganathan S, Chakrabarty B. Epilepsy, cognition and behavior. Indian J Pediatr. 2014. 81: 1056-62
8. Luan G, Li Y. Epilepsy surgery in China. Neurol Res. 2008. 30: 610-2
9. Luciana M. Cognitive development in children born preterm: Implications for theories of brain plasticity following early injury. Dev Psychopathol. 2003. 15: 1017-47
10. Schmeiser B, Zentner J, Steinhoff BJ, Brandt A, Schulze-Bonhage A, Kogias E. The role of presurgical EEG parameters and of reoperation for seizure outcome in temporal lobe epilepsy. Seizure. 2017. 51: 174-9
11. Skirrow C, Cross JH, Cormack F, Harkness W, Vargha-Khadem F, Baldeweg T. Long-term intellectual outcome after temporal lobe surgery in childhood. Neurology. 2011. 76: 1330-7
12. Styck KM, Watkins MW. Structural validity of the WISC-IV for students with ADHD. J Atten Disord. 2017. 21: 921-8
13. Usami K, Kubota M, Kawai K, Kunii N, Matsuo T, Ibayashi K. Long-term outcome and neuroradiologic changes after multiple hippocampal transection combined with multiple subpial transection or lesionectomy for temporal lobe epilepsy. Epilepsia. 2016. 57: 931-40
14. Vega-ZelayaL , Torres CV, Garnes-Camarena O. Electrocorticographic evidence and surgical implications of different physiopathologic subtypes of temporal epilepsy. Clin Neurophysiol. 2014. 125: 2349-57
15. Wirrell E, Wong-Kisiel L, Mandrekar J, Nickels K. Predictors and course of medically intractable epilepsy in young children presenting before 36 months of age: A retrospective, population-based study. Epilepsia. 2012. 53: 1563-9
16. Wirrell EC. Predicting pharmacoresistance in pediatric epilepsy. Epilepsia. 2013. 54: 19-22
17. Yang T, Hakimian S, Schwartz TH. Intraoperative ElectroCorticoGraphy (ECog): Indications, techniques, and utility in epilepsy surgery. Epileptic Disord. 2014. 16: 271-9
18. Yum MS, Ko TS, Lee JK, Hong S, Kim DS, Kim J. Surgical treatment for Ocalizationrelated infantile spasms: Excellent long-term outcomes. Clin Neurol Neurosurg. 2011. 113: 213-7