- Department of Neurosurgery, Medical University of South Carolina, Charleston, United States.
- Department of Neurosurgery, University of Texas at San Antonio, San Antonio, Texas, United States.
- College of Medicine, Medical University of South Carolina, Charleston, United States.
Correspondence Address:
Brian Fabian Saway, Department of Neurosurgery, Medical University of South Carolina, Charleston, United States.
DOI:10.25259/SNI_758_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Brian Fabian Saway1, Tristan Fielder2, Mohammed Abdul Alshareef1, Habib Emil Rafka3, Mithun Sattur1, Jonathan Lena1. Infratentorial retroclival and tentorial subdural hematoma from posterior communicating artery aneurysm rupture: A case report and systematic review of literature. 28-Oct-2022;13:499
How to cite this URL: Brian Fabian Saway1, Tristan Fielder2, Mohammed Abdul Alshareef1, Habib Emil Rafka3, Mithun Sattur1, Jonathan Lena1. Infratentorial retroclival and tentorial subdural hematoma from posterior communicating artery aneurysm rupture: A case report and systematic review of literature. 28-Oct-2022;13:499. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11955
Abstract
Background: The objective of this systematic review is to evaluate the pathogenesis, clinical course, and prognosis of patients who suffer from aneurysm rupture, leading to subdural hematoma (SDH) of the infratentorial space without associated subarachnoid hemorrhage (SAH).
Methods: Following Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, a literature review was conducted in PubMed and Scopus electronic databases for relevant published cases of aneurysmal SDH (AnSDH) of the infratentorial compartment without associated SAH. The presentation, treatment, clinical course, and outcome of identified cases are compiled. In addition, a patient suffering from an infratentorial SDH following aneurysm rupture is presented with an illustrative case.
Results: Three articles were identified and met inclusion criteria. All cases occurred from ruptured posterior communicating artery aneurysms. All patients arrived with a Hunt and Hess classification of 2 or less. Only one case was managed with operative aneurysm clipping and hematoma evacuation while the other three cases were managed endovascularly. There were no reported postoperative complications, vasospasm, or seizures reported. All patients had a final Modified Rankin score of 3 or less at last reported follow-up.
Conclusion: Infratentorial AnSDH without associated SAH is an etiology rarely reported in the literature. Here, we present a case report and systematic review demonstrating a relatively benign clinical course and outcome compared to report aneurysm rupture associated with SAH or mixed SAH and SDH. Moreover, there appear to be lower rates of vasospasm and improved outcomes in patients with isolated AnSDH compared to the literature aneurysmal SAH rates.
Keywords: Aneurysm, Embolization, Infratentorial, Subdural hematoma
INTRODUCTION
Acute subdural hematoma (SDH) typically results from traumatic brain injury. However, spontaneous SDH in the absence of trauma may result from neurovascular pathology. An example of neurovascular pathology resulting in spontaneous SDH, in the absence of trauma, is a ruptured intracranial aneurysm (RIA). While RIAs are commonly accompanied by subarachnoid hemorrhage (SAH), instances of aneurysms bleeding predominantly into the subdural space have been reported throughout the literature. These cases of aneurysmal SDH (AnSDH) involve a wide range of aneurysm locations across all major intracranial arterial distributions. Ruptured aneurysms of the posterior communicating segment of the internal carotid artery represent the most common etiology, leading to AnSDH. These commonly involve the supratentorial space and redistribute along the calvarial convexity and are accompanied by SAH. However, a pure acute SDH, without associated SAH, in the infratentorial compartment is very rare entity.[
MATERIALS AND METHODS
The presentation, treatment, clinical course, and outcome of a patient suffering from an infratentorial SDH following aneurysm rupture are presented with an illustrative case. Informed consent was obtained and all patient information was anonymized. Ethics approval was not required for this case. A literature review was conducted in PubMed and Scopus electronic databases from their dates of inception to December 2021 using combinations of the following keywords: “AnSDH” OR “AnSDH” OR “aneurysmal subdural” OR “aneurysm SDH” OR “aneurysm SDH” OR “aneurysm subdural” AND “infratentorial” OR “retroclival” OR “tentorial.” Only studies published in English or Spanish language were considered in this review. Our review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.[
CASE REPORT
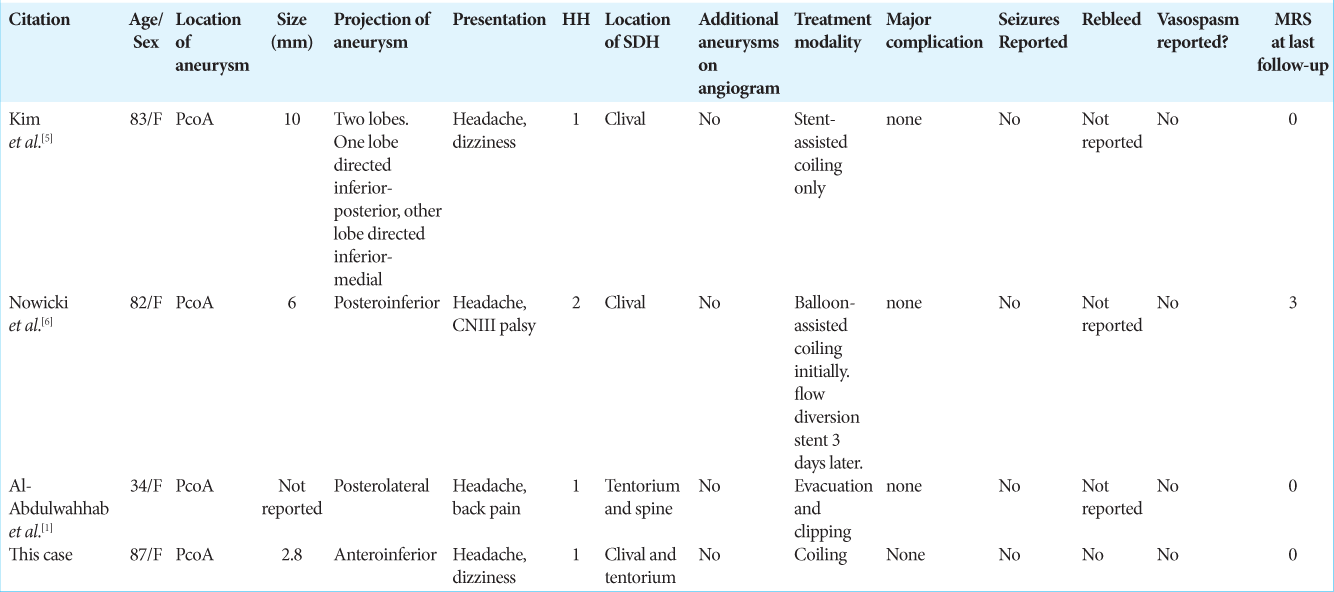
An 87-year-old female with a medical history of treated pancreatic cancer and on daily low-dose aspirin intake presented with sudden onset of severe, occipital headache with vertigo, and diaphoresis. She was awake and had mild right-sided sensorineural hearing loss. Computed tomography (CT) of the head without contrast demonstrated an acute SDH posterior to the clivus with extension into the upper cervical spine and along the tentorium bilaterally [
Figure 2:
Computed tomography (CT) without contrast of the head at initial presentation. (a) Coronal image with bilateral subdural hematoma (SDH) inferior to the tentorium with more blood product being on the right side. (b) Sagittal image with SDH inferior to the tentorium and dorsal to the clivus with extension into the ventral cervical spine.
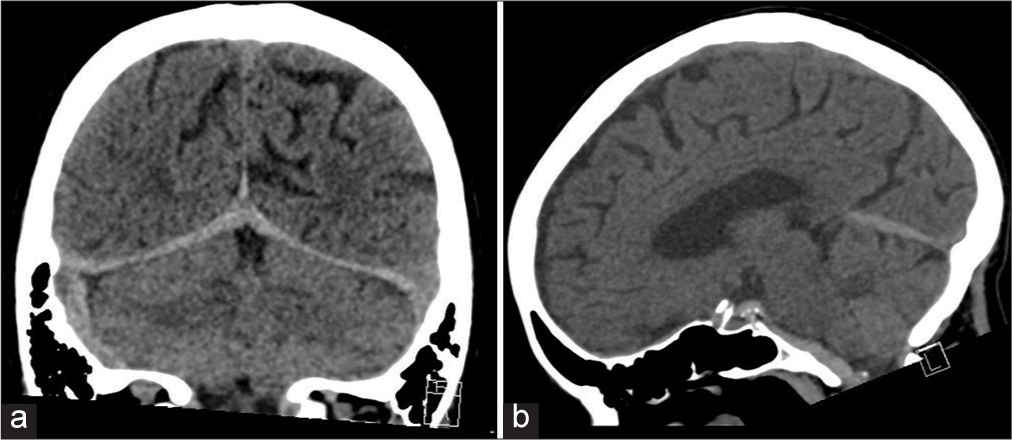
Figure 3:
Sagittal CT head without contrast (a) and CT angiogram (b) demonstrating SDH inferior to the tentorium with an inferiorly projecting posterior communicating artery (PComA) aneurysm (red arrow). This figure highlights the anatomical orientation of this aneurysm and how its rupture can lead to a pure SDH in the infratentorial compartment.
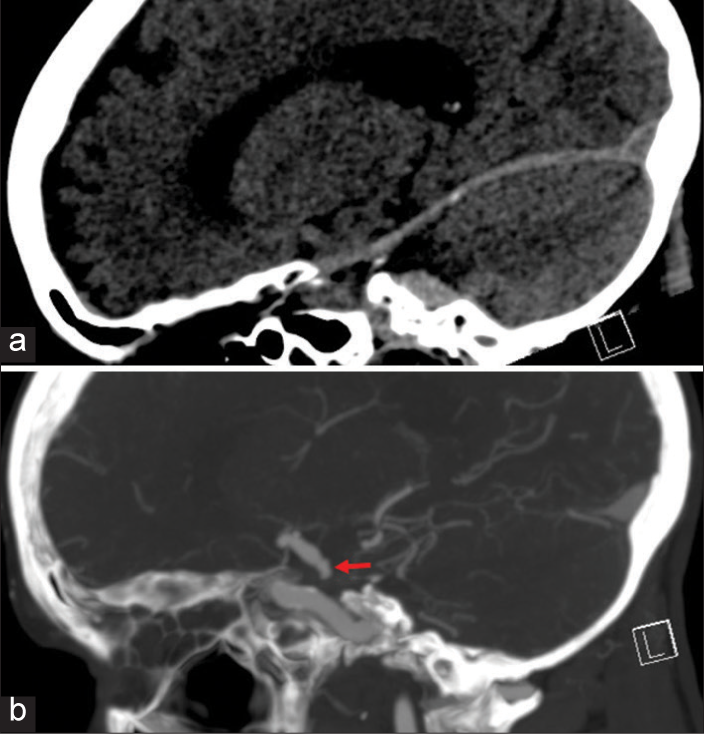
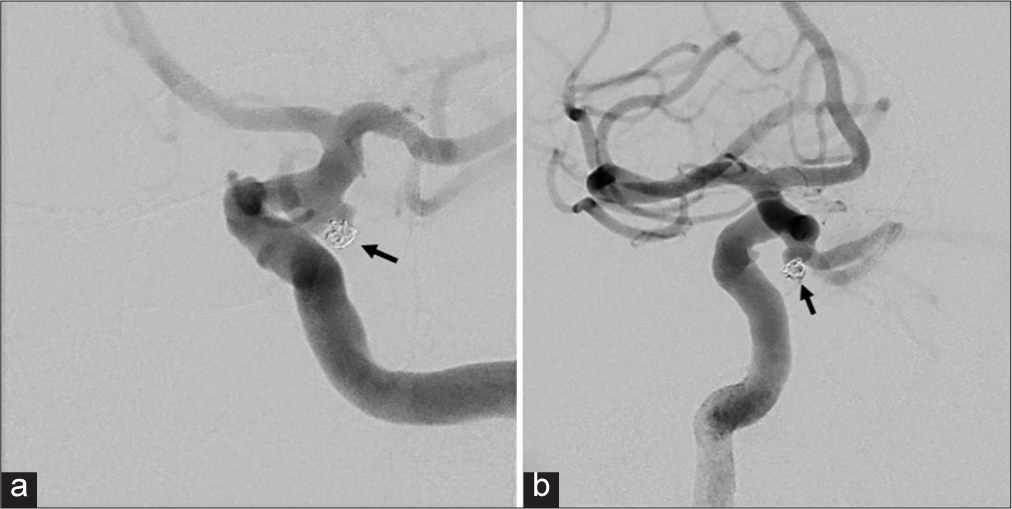
Figure 4:
Digital subtraction angiogram demonstrating orientation of aneurysm. (a) Anterior-posterior (AP) view demonstrates the AP aneurysm projection as the aneurysm (black arrow) is projecting parallel to ICA. (b) LAO view demonstrates the anterior-inferior projection of the aneurysm (white arrow).
DISCUSSION
Isolated AnSDH with no evidence of SAH is an exceptionally rare presentation and the incidence is not clearly defined. There are better estimates regarding mixed AnSDH and SAH. A recent series analyzed data from over 10,000 patients in the United States and showed the incidence of acute SDH among patients with nontraumatic aneurysmal SAH (aSAH) to be 3.5%.[
Hypotheses for SDH formation
Multiple hypotheses have been proposed but the most common involves the formation of adhesions between the aneurysmal wall, arachnoid membrane, and dura.[
Anatomy of dural folds and formation of SDH
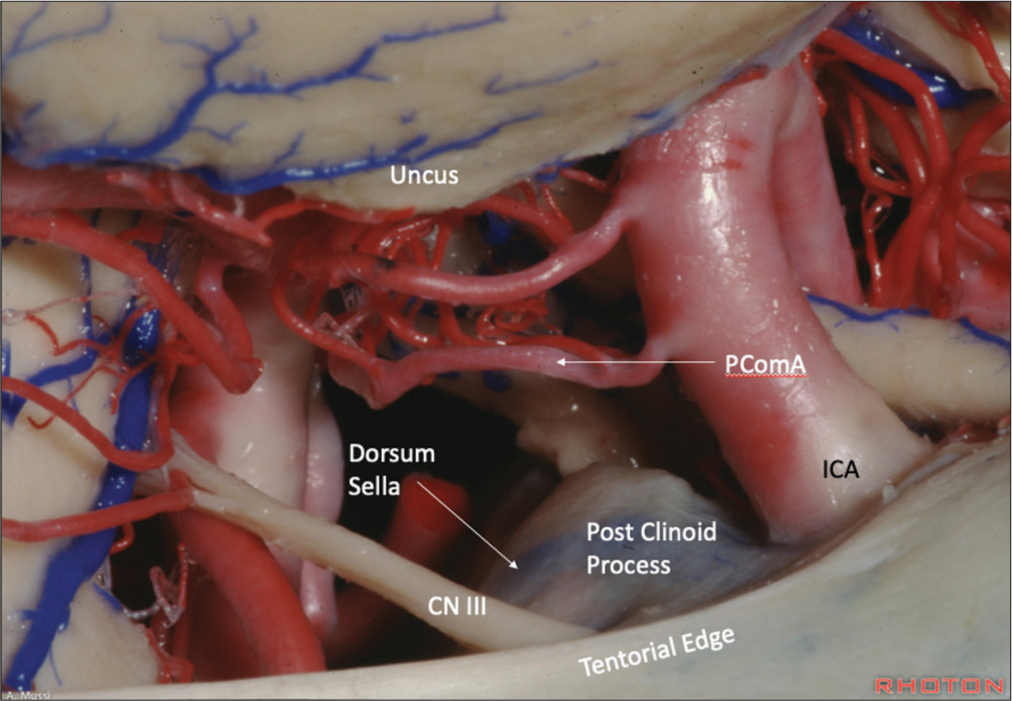
The intimate anatomy of aneurysms in the region of the ICA-Pcom to the posterior clinoid processes, dorsum sellae, interclinoid ligaments, petroclinoid ligaments, and adjacent tentorial edges presents the most plausible explanation for SDH formation [
Figure 6:
Cadaveric image from the Rhoton Collection demonstrating the anatomical proximity and relationship of the ICA-Pcom to the posterior clinoid processes, dorsum sellae, and adjacent tentorial edge. This provides for an anatomical understanding as to how a PComA rupture projecting inferiorly could lead to a SDH of the infratentorial compartment. Anatomical dissections performed by Antiono Mussi, MD, at Dr. Rhoton’s laboratory. Reproduced with permission from the Rhoton Collection (
Outcome data
There are no large studies analyzing outcomes of patients with isolated AnSDH, but few larger studies investigating the outcomes of patients with mixed AnSDH are present in the literature. Kaur et al.’s study reported that patients with aSAH and acute SDH had almost double the mortality when compared to patients with only aSAH (24% vs. 12%, respectively).[
Given this current review and the present case, several points may be made. Primarily, the patients presenting with aneurysm rupture with SDH formation in the absence of SAH in the infratentorial compartment may likely have a better outcome with a close return to function with the aneurysm adequately secured. Second, the presence of a spontaneous and acute infratentorial SDH without obvious traumatic etiology is rare, and an underlying cerebrovascular pathology such as an aneurysm must be ruled out. From our experience with this case and the subsequent review of literature, we recommend the use of CT angiogram in the work-up to rule out an aneurysmal etiology. We also recommend considering the use of digital subtraction angiography if the CT angiogram is found to be negative. Finally, this review provides support that vasospasm in this subset of aneurysm rupture may occur less frequently and thus not require aggressive monitoring and prophylaxis as compared to aSAH and mixed SAH with SDH.
CONCLUSION
Infratentorial AnSDH without associated SAH is an etiology rarely reported in the literature. Here, we present a case report and review of the literature demonstrating a relatively benign clinical course and outcome compared to reported aneurysm rupture associated with SAH or mixed SAH and SDD. Moreover, there appear to be lower rates of vasospasm and improved outcomes in patients with isolated AnSDH compared to the literature aSAH rates.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Al-Abdulwahhab AH, Al-Sharydah AM, Al-Suhibani SS, Almulhim AS, Al-Dhafeeri OM, Al-Jubran SA. A ruptured posterior communicating artery aneurysm presenting as tentorial and spinal isolated subdural hemorrhage: A case report and literature review. BMC Neurol. 2020. 20: 102
2. Biesbroek JM, Rinkel GJ, Algra A, van der Sprenkel JW. Risk factors for acute subdural hematoma from intracranial aneurysm rupture. Neurosurgery. 2012. 71: 268-9
3. Kondziolka D, Bernstein M, ter Brugge K, Schutz H. Acute subdural hematoma from ruptured posterior communicating artery aneurysm. Neurosurgery. 1988. 22: 151-4
4. Kaur G, Dakay K, Sursal T, Pisapia J, Bowers C, Hanft S. Acute subdural hematomas secondary to aneurysmal subarachnoid hemorrhage confer poor prognosis: A national perspective. J Neurointerv Surg. 2021. 13: 426-9
5. Kim MS, Jung JR, Yoon SW, Lee CH. Subdural hematoma of the posterior fossa due to posterior communicating artery aneurysm rupture. Surg Neurol Int. 2012. 3: 39
6. Nowicki JL, Agzarian M, Chryssidis S, Harding M. Pure retroclival subdural hemorrhage secondary to ruptured posterior communicating artery aneurysm. Br J Neurosurg. 2020. p. 1-4
7. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021. 372: n71
8. Park SM, Han YM, Park YS, Park IS, Baik MW, Yang JH. Acute aneurysmal subdural hematoma: Clinical and radiological characteristics. J Korean Neurosurg Soc. 2005. 37: 329-35
9. Rhoton AL, editors. Cranial Anatomy and Surgical Approaches. Philadelphia, PA: Lippincott Williams and Wilkins; 2003. p.
10. Schuss P, Konczalla J, Platz J, Vatter H, Seifert V, Güresir E. Aneurysm-related subarachnoid hemorrhage and acute subdural hematoma: Single-center series and systematic review. J Neurosurg. 2013. 118: 984-90