- Department of Anesthesia, Neuroanesthesia Cell, Indira Gandhi Medical College, Shimla, Himachal Pradesh, India
- Department of Anesthesia and Critical Care, Neuroanesthesia Division Under, Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, India
Correspondence Address:
Kunal Kumar Sharma, Department of Anesthesia, Neuroanesthesia Cell Under, Indira Gandhi Medical College, Shimla, Himachal Pradesh, India.
DOI:10.25259/SNI_934_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sharma KK1, Chauhan B2. Integrated dexmedetomidine-sevoflurane algorithm for anesthetic induction – A viable asset for neurosurgery. Surg Neurol Int 06-Dec-2024;15:455
How to cite this URL: Sharma KK1, Chauhan B2. Integrated dexmedetomidine-sevoflurane algorithm for anesthetic induction – A viable asset for neurosurgery. Surg Neurol Int 06-Dec-2024;15:455. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13267
Dear Editor,
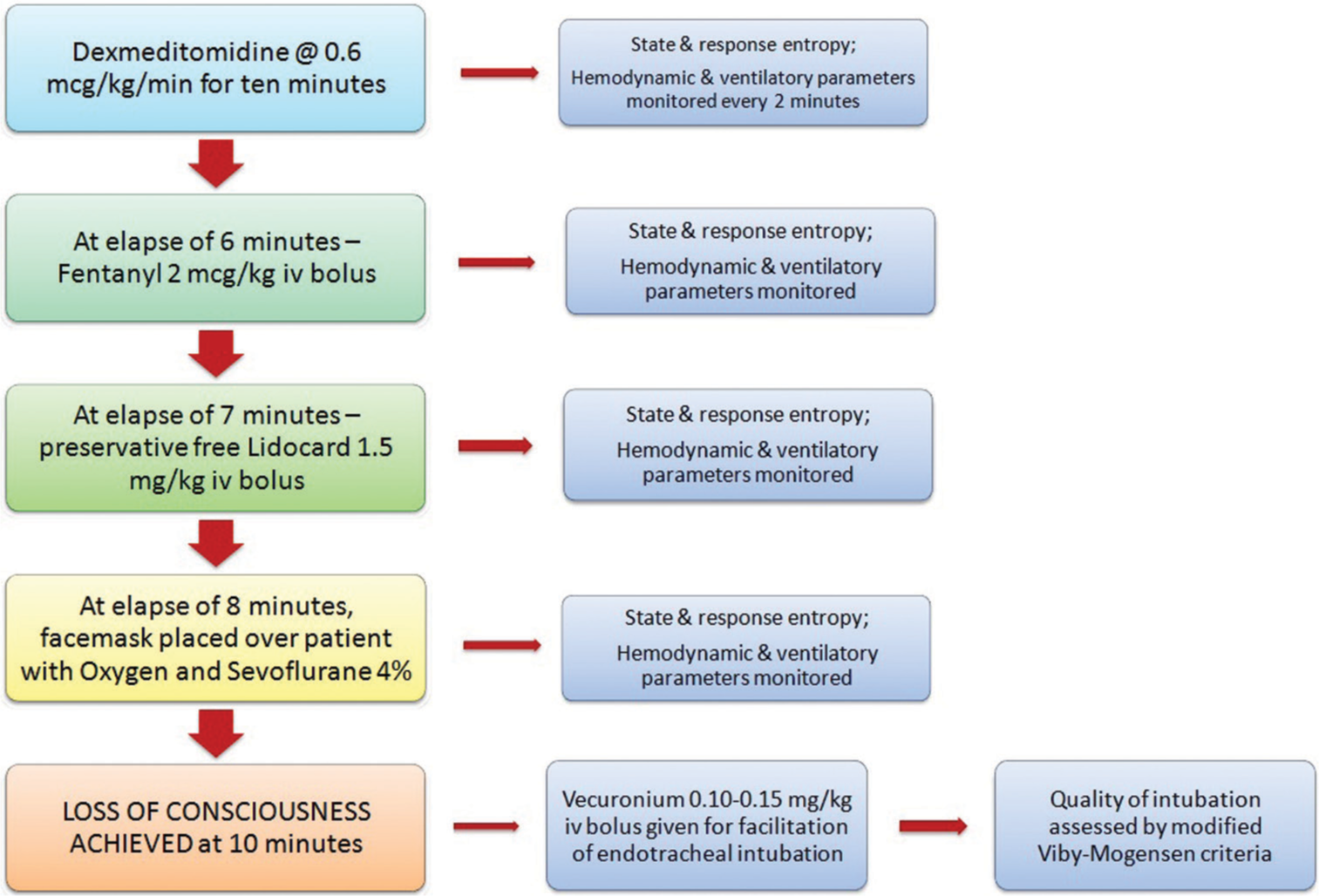
As per the existing literature, there is established evidence against the usage of propofol, thiopentone, and etomidate for anesthetic induction in patients with mitochondrial dysfunction.[
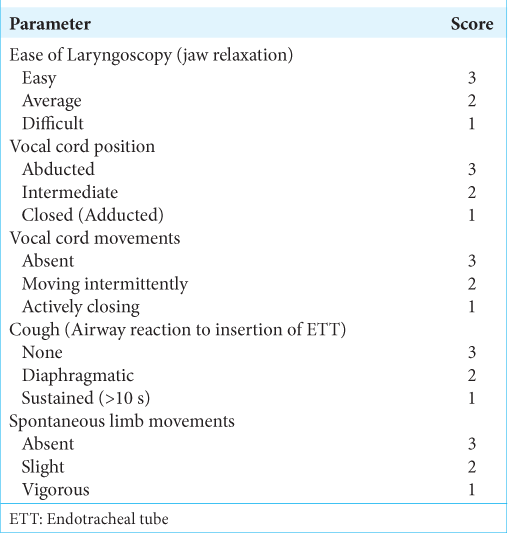
Table 2:
Quality of intubation criteria.[ 11 ]
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Acco A, Comar JF, Bracht A. Metabolic effects of propofol in the isolated perfused rat liver. Basic Clin Pharmacol Toxicol. 2004. 95: 166-74
2. Can M, Gul S, Bektas S, Hanci V, Acikgoz S. Effects of dexmedetomidine or methylprednisolone on inflammatory responses in spinal cord injury. Acta Anaesth Scand. 2009. 53: 1068-72
3. Driessen J, Willems S, Dercksen S, Giele J, van der Staak F, Smeitink J. Anesthesia-related morbidity and mortality after surgery for muscle biopsy in children with mitochondrial defects. Paediatr Anaesth. 2007. 17: 16-21
4. Gao J, Sun Z, Xiao Z, Du Q, Niu X, Wang G. Dexmedetomidine modulates neuroinflammation and improves outcome via alpha2-adrenergic receptor signaling after rat spinal cord injury. Br J Anaesth. 2019. 123: 827-38
5. He H, Zhou Y, Zhou Y, Zhuang J, He X, Wang S. Dexmedetomidine mitigates microglia-mediated neuroinflammation through upregulation of programmed cell death protein 1 in a rat spinal cord injury model. J Neurotrauma. 2018. 35: 2591-603
6. Liang Y, Huang Y, Shao R, Xiao F, Lin F, Dai H. Propofol produces neurotoxicity by inducing mitochondrial apoptosis. Exp Ther Med. 2022. 24: 630
7. Mu B, Xu W, Li H, Suo Z, Wang X, Zheng Y. Determination of the effective dose of dexmedetomidine to achieve loss of consciousness during anesthesia induction. Front Med. 2023. 10: 1158085
8. Ramsay MA, Luterman DL. Dexmedetomidine as a total intravenous anesthetic agent. Anesthesiology. 2004. 101: 787-90
9. Rigoulet M, Devin A, Avéret N, Vandais B, Guérin B. Mechanisms of inhibition and uncoupling of respiration in isolated rat liver mitochondria by the general anesthetic 2,6-diisopropylphenol. Eur J Biochem. 1996. 241: 280-5
10. Schwarz A, Nossaman B, Carollo D, Ramadhyani U. Dexmedetomidine for neurosurgical procedures. Curr Anesthesiol Rep. 2013. 3: 205-9
11. Sun L, Niu K, Guo J, Tu J, Ma B, An J. Dexmedetomidine attenuates postoperative spatial memory impairment after surgery by reducing cytochrome C. BMC Anesthesiol. 2023. 23: 85
12. Viby-Mogensen J, Engbaek J, Eriksson LI, Gramstad L, Jensen E, Jensen FS. Good clinical research practice (GCRP) in pharmacodynamic studies of neuromuscular blocking agents. Acta Anaesthesiol Scand. 1996. 40: 59-74