- Department of Neurosurgery, Kumamoto Rosai Hospital, 1670 Takehara-Cho, Yatsushiro-Shi,
- Department of Neurosurgery, Amakusa Regional Medical Center, 854-1 Shokuba, Kameba-Cho, Amakusa,
- Department of Neurosurgery, Minamata City General Hospital and Medical Center, 1-2-1 Tenjin-Cho, Minamata,
- Department of Neurosurgery, Kumamoto University School of Medicine, 1-1-1 Honjo, Chuo-Ku, Kumamoto, Japan.
Correspondence Address:
Hiroki Uchikawa
Department of Neurosurgery, Kumamoto University School of Medicine, 1-1-1 Honjo, Chuo-Ku, Kumamoto, Japan.
DOI:10.25259/SNI_236_2019
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hiroki Uchikawa, Shigeo Yamashiro, Yasuyuki Hitoshi, Makoto Yoshikawa, Akimasa Yoshida, Shigetoshi Yano. Interval between endoscopic surgery and decreased intracranial pressure related to putaminal hemorrhage prognosis. 25-Apr-2020;11:78
How to cite this URL: Hiroki Uchikawa, Shigeo Yamashiro, Yasuyuki Hitoshi, Makoto Yoshikawa, Akimasa Yoshida, Shigetoshi Yano. Interval between endoscopic surgery and decreased intracranial pressure related to putaminal hemorrhage prognosis. 25-Apr-2020;11:78. Available from: https://surgicalneurologyint.com/surgicalint-articles/9975/
Abstract
Background: Endoscopic evacuation of a putaminal hemorrhage is effective and minimally invasive; however, it may not result in sufficient brain decompression. While monitoring postoperative intracranial pressure (ICP) is likely useful, specific ICP data in patients with a putaminal hemorrhage are limited. The aim of this study was to determine the association between postoperative ICP and the prognosis of patients with putaminal hemorrhage after endoscopic surgery.
Methods: We retrospectively analyzed 24 consecutive patients with a putaminal hemorrhage in whom ICP monitoring after endoscopic surgery was performed. Clinical data regarding hematoma volume, evacuation rate, onset-to-treatment time, operation time, ICP max, ICP peak out time (T peak out), and neurological outcomes on discharge were investigated.
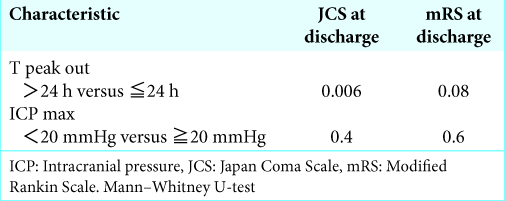
Results: From August 2011 to October 2015, 24 patients with a putaminal hemorrhage were analyzed. Consciousness on admission and hemorrhage volume were associated with poor outcomes after endoscopic surgery for putaminal hemorrhage. The hematoma volume, operation time, and evacuation rate of hemorrhage were correlated to early peak out of ICP. Furthermore, a T peak out ≤24 h was significantly associated with good neurological outcomes on discharge.
Conclusions: Our data suggest that early peak out (≤24 h) of ICP after endoscopic surgery is predictive of a good prognosis following putaminal hemorrhage. Operation time and evacuation rate of hemorrhage could hasten peak out of ICP and improve outcomes in patients with a putaminal hemorrhage after endoscopic surgery.
Keywords: Endoscopic surgery, Intracranial pressure, Putaminal hemorrhage
INTRODUCTION
A putaminal hemorrhage is the most frequent subtype of spontaneous cerebral hemorrhage and accounts for approximately 40% of cerebral hemorrhages. Putaminal hemorrhage often requires the surgical evacuation of a hematoma, which is performed using various surgical procedures, such as conventional craniotomy, stereotactic aspiration, and endoscopic surgery.
The advantages of surgical treatment for putaminal hemorrhage have previously been reported.[
MATERIALS AND METHODS
Patients
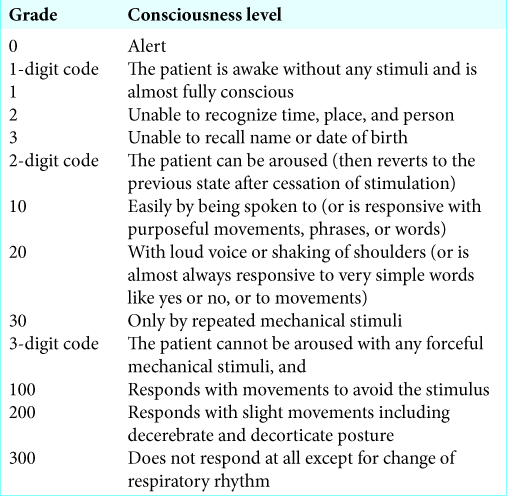
We retrospectively analyzed 25 consecutive patients with putaminal hemorrhage treated by endoscopic surgery in our hospital from August 2011 to September 2015 who underwent postoperative ICP monitoring using the Codman ICP Microsensor (Codman Neuro, USA). Surgical indications were a hematoma volume of greater than 30 mL and impaired consciousness due to a hematoma. Surgical hematoma evacuation was performed within 24 h after onset. ICP was recorded every 1 h in all patients after endoscopic surgery. The following clinical characteristics and parameters were reviewed: sex, age, preoperative Japan Coma Scale (JCS), hematoma volume, evacuation rate of hematoma, onset-to- treatment time, operation time, maximum of ICP, T peak out of ICP (T peak out), and neurological outcome. T peak out was defined as follows; the time from completion of surgery until ICP began to decrease, specifically, it was defined as the time until ICP fell below the ICP max – 2 mmHg and did not become elevated to the same value again. Since T peak out is subjective, it was measured several times by observers who were blinded to parameters and outcomes. Neurological outcomes were evaluated using the JCS [
Great efforts were made to obtain written consent from all patients and their families for this study after providing them with general instructions, and our own institutional ethics committee reviewed this study thoroughly and approved it in advance.
Clinical management
Surgical indications were as above. Soon after diagnosis, acute hypertension was treated to achieve systolic blood pressure below 140 mmHg with intravenous infusion of a calcium channel blocker. Endoscopic surgery was performed as soon as possible under general anesthesia in principle, but local anesthesia was considered for comatose patients with brain herniations who were unable to delay treatment for induction of general anesthesia. We used a 2.7 mm rigid endoscope (Olympus Corp., Tokyo) and Neuroport (Olympus Corp.). A linear skin incision and a burr hole were made on the forehead, and the hematoma was evacuated through the burr hole with a trans-frontal lobe approach. An ICP transducer was inserted into the subcortex from the tract of the endoscope. Cerebrospinal fluid drainage was performed if needed after endoscopic surgery. All patients received critical care in the intensive care unit with ICP monitoring. Intracranial hypertension was first treated using a noninvasive procedure, such as head elevation, osmotic diuretics, hyperventilation, or sedation. However, if these methods were not effective, cerebrospinal fluid drainage was performed. If necessary, re- operation or decompressive craniectomy was considered.
Radiological analysis
An initial computed tomography (CT) scan was obtained in all patients, and the diameter and hematoma volume were assessed. Hematoma volume was calculated from preoperative CT scans using the a × b × c × 0.5 method.[
Statistical analysis
Statistical analyses were conducted using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which are a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander designed to add statistical functions that are frequently used in biostatistics. Statistical significance was assumed for P < 0.05. Values are presented as the mean ± standard deviation. We analyzed data using the Mann–Whitney U-test and Spearman’s rank correlation coefficient.
RESULTS
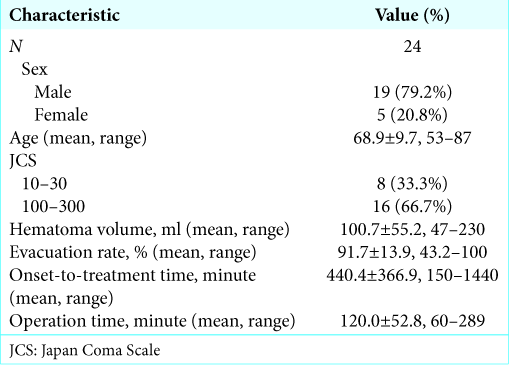
One patient died because of acute rebleeding just after surgery and was excluded from this study. Effective ICP monitoring was performed in 24 patients (19 male patients, 5 female patients; mean age: 68.9 years; and age range: 53– 87 years) with putaminal hemorrhage treated by endoscopic surgery. The demographic and clinical characteristics of patients included in our study are shown in
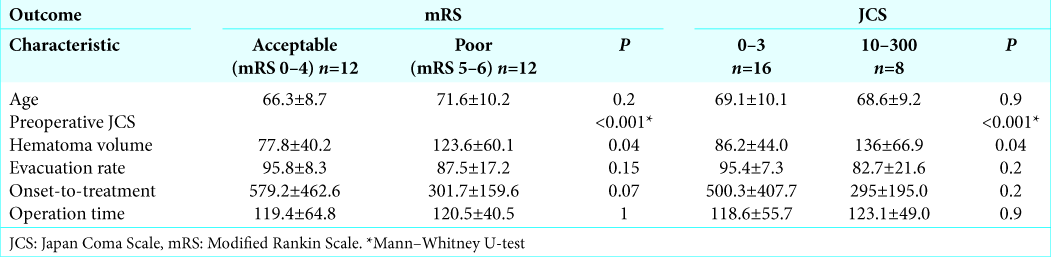
The relationship between preoperative parameters and neurological outcomes is shown in
DISCUSSION
Hematoma evacuation for putaminal hemorrhage is a well- established surgery, and many cases have been treated in the world. In recent years, endoscopic evacuation of cerebral hematomas has become more common worldwide.[
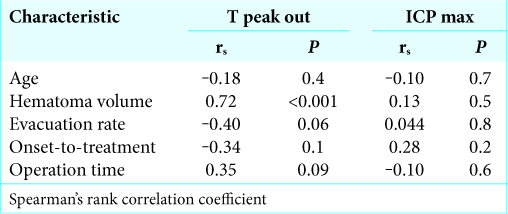
Among preoperative parameters, hematoma volume is strongly correlated with T peak out. This follows logically from routine clinical observations in which a large cerebral hemorrhage has been shown to inflict broad damage on the brain by prolonging cerebral edema and delaying the time of peak out of ICP. Furthermore, the evacuation rate and operation time were weakly correlated with T peak out. In addition, residual hematoma after operation might stimulate the surrounding brain and lead to prolonged cerebral edema. Meanwhile, an efficient surgery should result in less damage to the surrounding brain. None of the preoperative parameters were correlated with ICP max. The maximum value of ICP appeared to be less affected by preoperative parameters.
There are some reports demonstrating an association between ICP and mortality or neurological outcomes.[
There are few limitations to the present study. First, the present study analyzed a small number of cases. In addition, it was a retrospective observational study at a single center. While we found that the early peak out of ICP was reliably predictive of a good prognosis in patients with putaminal hemorrhage who were treated using endoscopic surgery, it remains unclear whether intentional early peak out of ICP by aggressive intracranial hypotension therapy is associated with a good prognosis. At present, it is unknown whether intensive lowering of ICP to achieve early peak out improves prognosis. Further large prospective interventional studies may be needed. Second, the cases included in this study include only those patients with a putaminal hemorrhage who were treated using endoscopic surgery. Therefore, generalizing our findings requires further research. Nevertheless, our study demonstrates that ICP monitoring and T peak out analysis may be helpful for other types of cerebral hemorrhage. Third, neurological outcomes were only measured at the time of discharge, and it is possible that only short-term prognosis is shown. It is necessary to investigate the long-term prognosis after 3 months or 1 year.
In addition, we did not use detailed neurological outcomes, such as the Glasgow Coma Scale or National Institute of Health Stroke Scale. Finally, as described above, the T peak out tends to be subjective; however, this measurement was performed several times by investigators who were blinded to any parameters or outcomes.
The results of this study suggested that monitoring postoperative ICP after endoscopic surgery is useful for predicting prognosis in patients with a putaminal hemorrhage. Early peak out of ICP might contribute to a good prognosis in patients with a putaminal hemorrhage after endoscopic surgery. Although additional studies that validate our findings are needed, ICP monitoring for patients with cerebral hemorrhage after surgery can be a promising supplementary method in the future.
CONCLUSION
The results of this study suggested that monitoring postoperative ICP after endoscopic surgery is useful for predicting prognosis in patients with a putaminal hemorrhage. Early peak out of ICP might contribute to a good prognosis in patients with a putaminal hemorrhage after endoscopic surgery. Although additional studies that validate our findings are needed, ICP monitoring for patients with cerebral hemorrhage after surgery can be a promising supplementary method in the future.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing.
References
1. Alali AS, Fowler RA, Mainprize TG, Scales DC, Kiss A, de Mestral C. Intracranial pressure monitoring in severe traumatic brain injury: Results from the American college of surgeons trauma quality improvement program. J Neurotrauma. 2013. 30: 1737-46
2. Auer LM, Deinsberger W, Niederkorn K, Gell G, Kleinert R, Schneider G. Endoscopic surgery versus medical treatment for spontaneous intracerebral hematoma: A randomized study. J Neurosurg. 1989. 70: 530-5
3. Cho DY, Chen CC, Chang CS, Lee WY, Tso M. Endoscopic surgery for spontaneous basal ganglia hemorrhage: Comparing endoscopic surgery, stereotactic aspiration, and craniotomy in noncomatose patients. Surg Neuro. 2006. 65: 547-55
4. Farahvar A, Gerber LM, Chiu YL, Carney N, Härtl R, Ghajar J. Increased mortality in patients with severe traumatic brain injury treated without intracranial pressure monitoring. J Neurosurg. 2012. 117: 729-34
5. Fernandes HM, Siddique S, Banister K, Chambers I, Wooldridge T, Gregson B. Continuous monitoring of ICP and CPP following ICH and its relationship to clinical, radiological and surgical parameters. Acta Neurochir Suppl. 2000. 76: 463-6
6. Gerber LM, Chiu YL, Carney N, Härtl R, Ghajar J. Marked reduction in mortality in patients with severe traumatic brain injury. J Neurosurg. 2013. 119: 1583-90
7. Hattori N, Katayama Y, Maya Y, Gatherer A. Impact of stereotactic hematoma evacuation on activities of daily living during the chronic period following spontaneous putaminal hemorrhage: A randomized study. J Neurosurg. 2004. 101: 417-20
8. Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996. 27: 1304-5
9. Nagasaka T, Tsugeno M, Ikeda H, Okamoto T, Inao S, Wakabayashi T. Early recovery and better evacuation rate in neuroendoscopic surgery for spontaneous intracerebral hemorrhage using a multifunctional cannula: Preliminary study in comparison with craniotomy. J Stroke Cerebrovasc Dis. 2011. 20: 208-13
10. Nikaina I, Paterakis K, Paraforos G, Dardiotis E, Chovas A, Papadopoulos D. Cerebral perfusion pressure, microdialysis biochemistry, and clinical outcome in patients with spontaneous intracerebral hematomas. J Crit Care. 2012. 27: 83-8
11. Nishihara T, Morita A, Teraoka A, Kirino T. Endoscopy-guided removal of spontaneous intracerebral hemorrhage: Comparison with computer tomography-guided stereotactic evacuation. Childs Nerv Syst. 2007. 23: 677-83
12. Pantazis G, Tsitsopoulos P, Mihas C, Katsiva V, Stavrianos V, Zymaris S. Early surgical treatment vs conservative management for spontaneous supratentorial intracerebral hematomas: A prospective randomized study. Surg Neurol. 2006. 66: 492-501
13. Sykora M, Steinmacher S, Steiner T, Poli S, Diedler J. Association of intracranial pressure with outcome in comatose patients with intracerebral hemorrhage. J Neurol Sci. 2014. 342: 141-5
14. Tian Y, Wang Z, Jia Y, Li S, Wang B, Wang S. Intracranial pressure variability predicts short-term outcome after intracerebral hemorrhage: A retrospective study. J Neurol Sci. 2013. 330: 38-44
15. Xu X, Chen X, Li F, Zheng X, Wang Q, Sun G. Effectiveness of endoscopic surgery for supratentorial hypertensive intracerebral hemorrhage: A comparison with craniotomy. J Neurosurg. 2018. 128: 553-9