- Department of Neurosurgery, Penn State Hershey Medical Center, Hershey, Pennsylvania, United States
- Department of Neurological Surgery, Indiana University School of Medicine, Section of Pediatric Neurosurgery, Riley Hospital for Children, Goodman Campbell Brain and Spine, Indianapolis, Indiana, United States
- Department of Neurosurgery, Baylor College of Medicine, Division of Pediatric Neurosurgery, Texas Children's Hospital, Houston, Texas, United States
Correspondence Address:
Sandi Lam
Department of Neurosurgery, Baylor College of Medicine, Division of Pediatric Neurosurgery, Texas Children's Hospital, Houston, Texas, United States
DOI:10.4103/sni.sni_320_18
Copyright: © 2019 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Ryan Jafrani, Jeffrey S. Raskin, Ascher Kaufman, Sandi Lam. Intracranial arachnoid cysts: Pediatric neurosurgery update. 06-Feb-2019;10:15
How to cite this URL: Ryan Jafrani, Jeffrey S. Raskin, Ascher Kaufman, Sandi Lam. Intracranial arachnoid cysts: Pediatric neurosurgery update. 06-Feb-2019;10:15. Available from: http://surgicalneurologyint.com/surgicalint-articles/9199/
Abstract
Background:With the greater worldwide availability of neuroimaging, more intracranial arachnoid cysts (IACs) are being found in all age groups. A subset of these lesions become symptomatic and requires neurosurgical management. The clinical presentations of IACs vary from asymptomatic to extremely symptomatic. Here, we reviewed the clinical presentation and treatment considerations for pediatric IACs.
Case Description:Here, we presented three cases of IAC, focusing on different clinical and treatment considerations.
Conclusion:IACs can be challenging to manage. There is no Class I Evidence to guide how these should be treated. We suggest clinical decision-making framework as to how to treat IACs based on our understanding of the natural history, risks/benefits of treatments, and outcomes in the future, require better patient selection for the surgical management of IACs will be warranted.
Keywords: Arachnoid cyst, neurosurgery, pediatric
INTRODUCTION
Richard Bright first reported medical cases in 1831 involving intracranial arachnoid cysts (IACs). These were found in patients from all age groups but mostly (75%) occurred in children.[
DIAGNOSTIC AND THERAPEUTIC CONSIDERATIONS FOR IACS
The growing availability of neuroimaging (MR, CT) worldwide has increased the frequency with which IACs have been diagnosed. With CT cisternography, they may be defined as communicating or noncommunicating with the subarachnoid space.[
EPIDEMIOLOGY
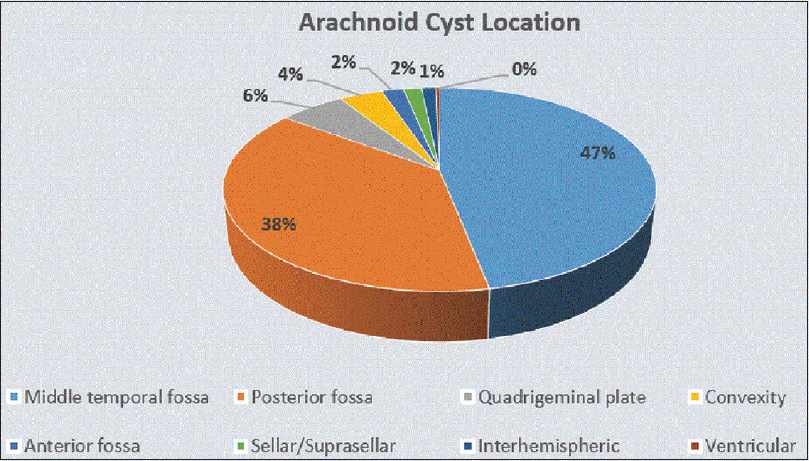
IACs occur in 2.6%of children and 1.4% of adults.[
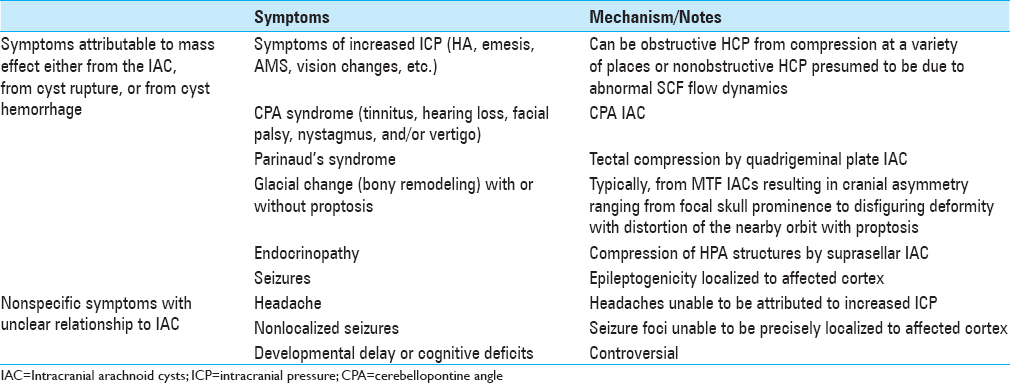
Figure 1
Large arachnoid cyst occupying majority left anterior and middle cranial fossae (Case 1). (a–c) Axial, coronal, and sagittal, respectively; coronal T2-weighted magnetic resonance imaging (MRI) of the brain demonstrating an arachnoid cyst eliciting extensive mass effect and 1 cm rightward midline shift. (d–f) Corresponding T2-weighted MRI 8 months postplacement of cystoperitoneal shunt demonstrating interval reduction of cyst size and corresponding midline shift
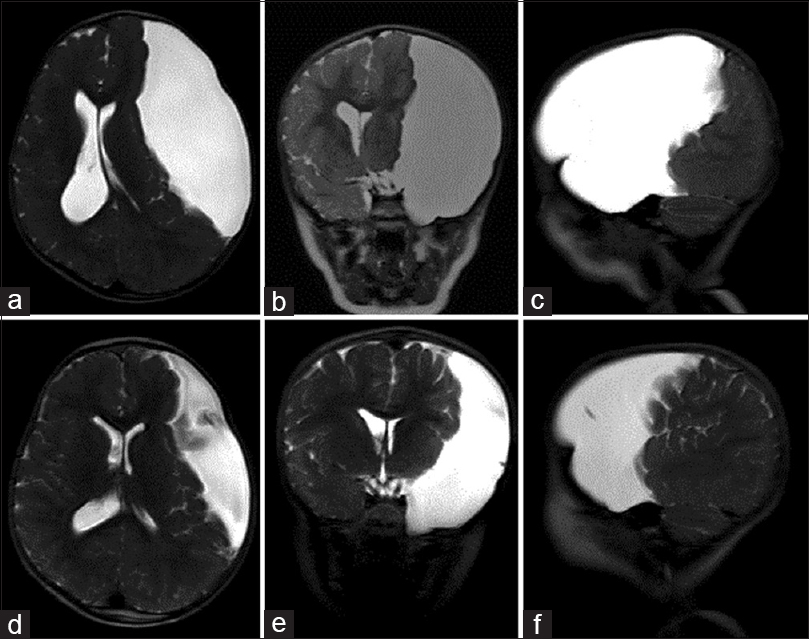
Figure 2
Large left middle cranial fossa arachnoid cyst (Case 2). (a and b) Axial and sagittal T1-weighted magnetic resonance imaging (MRI) of the brain demonstrating an arachnoid cyst eliciting extensive mass effect with sphenoid wing remodeling and distal left MCA branch displacement. (c and d) Axial and sagittal T1-weighted MRI of the brain 2 years after arachnoid cyst fenestration into the basal cisterns, displaying interval reduction in cyst size and decreased mass effect
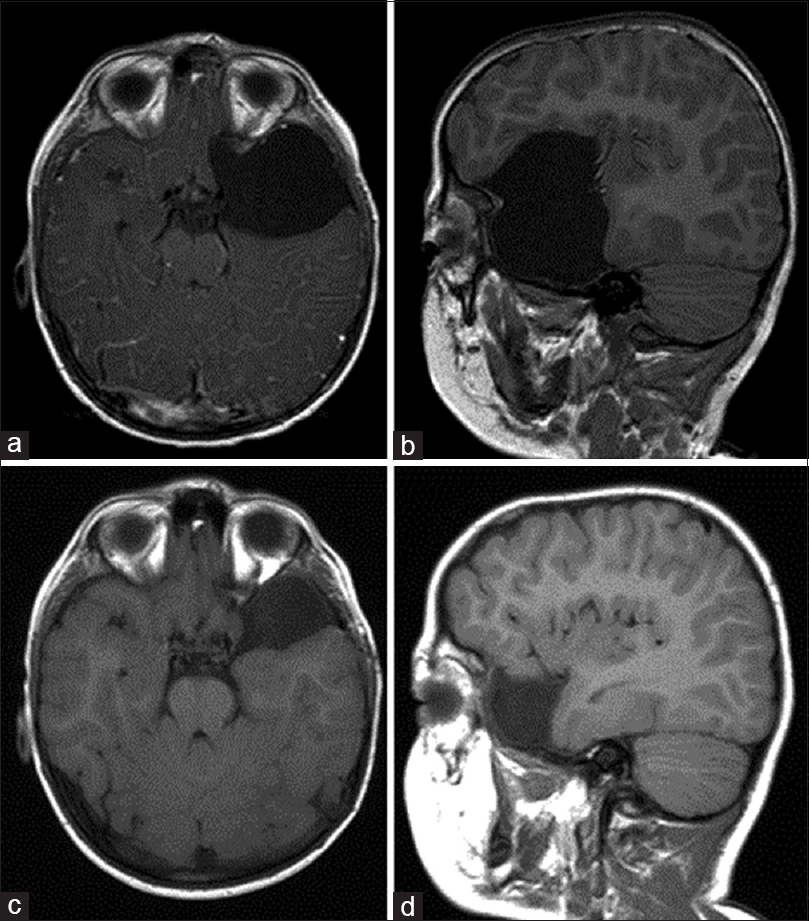
Figure 3
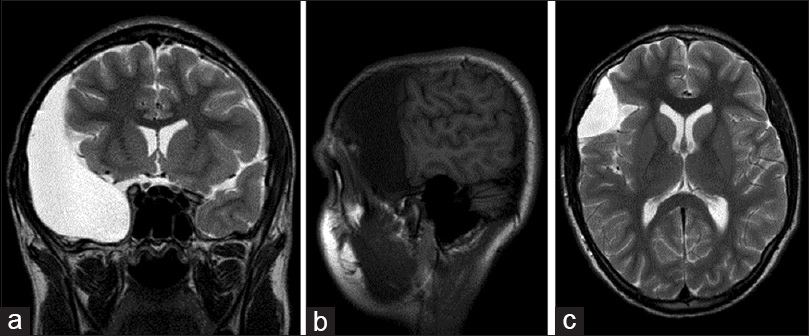
Previously asymptomatic right middle and anterior cranial fossae archnoid cyst with subdural fluid collection (Case 3). (a and b) Coronal and sagittal T1-weighted magnetic resonance imaging (MRI) performed secondary to trauma demonstrated a previously asymptomatic archnoid cyst of the right anterior an middle cranial fossae. (c) Axial T2 MRI revealed small bilateral hemisphere CSF-isointense subdural fluid collections suggestive of cyst rupture. (d–f) Coronal, sagittal, and axial T2-weighted MRI performed on presentation to the emergency department 3 weeks after initial trial of conservative treatment. Interval development of extensive right hemisphere hygroma was seen producing midline shift with compression of the lateral ventricle
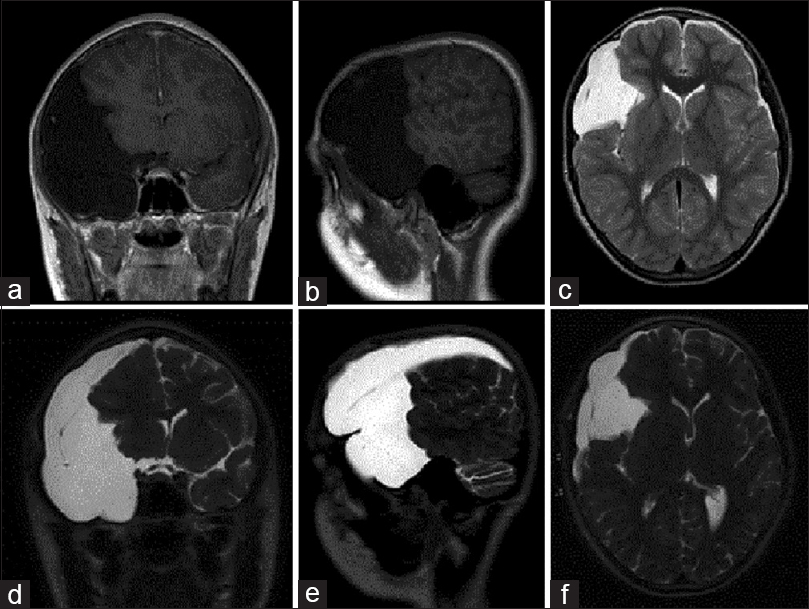
Figure 4
Right middle and anterior fossae arachnoid cyst with surgical resolution of traumatic hygroma (Case 3). Despite attempted management of hygroma development with cystoperitoneal shunt, symptoms persisted necessitating surgical fenestration of cyst walls, and regular follow-up was maintained. (a) Coronal T2-weighted magnetic resonance imaging (MRI) of the brain performed at 3 years postoperative follow-up demonstrated reabsorption of right hemisphere hygroma. (b) Sagittal T1-weighted MRI revealed interval reduction in arachnoid cyst-induced mass effect with reexpansion of cerebral sulci. (c) Axial T2-weighted MRI illustrated resolution of midline shift and relief of compression of the lateral ventricle
ETIOLOGY/PATHOGENESIS
IACs are congenital lesions, of unknown etiology. Previously, they were thought to result from gestational ischemic, traumatic, or infectious insults; these theories are no longer supported.[
HISTOPATHOLOGY
Histopathologically, the arachnoid cyst (AC) wall consists of duplicated layers of normal arachnoid. However, ultrastructural examination demonstrates a split layer of abnormal arachnoid tissue characterized by hyperplastic arachnoid cells, increased collagen, and absence of the spider-like trabeculations characteristic of normal arachnoid.[
MECHANISMS OF IAC EXPANSION
Proposed mechanisms for IAC expansion include fluid diffusion down an osmotic gradient,[
CT AND MR-IMAGING CHARACTERISTICS OF IACS
CT and MR studies demonstrate that IACs are well-circumscribed, extra-axial, simple cystic lesions. They are isodense to CSF on CT, and isointense to CSF on all magnetic resonance image (MRI) sequences. Unlike dermoid or epidermoid cysts, they do not exhibit diffusion restriction on MRI and are not lobulated with heterogonous signal characteristic on MRI FLAIR imaging. Ruptured ACs can fill with blood products, resulting in imaging studies reflecting progressive blood degradation pathways. Chronic subdural hematomas or subdural hygromas can be isodense to CSF on CT. However, on MRI, they do not share signal characteristics of CSF. Rather they demonstrate enhancing membranes and morphology distinct from ACs (i.e., crescent-shaped layering along the cerebral convexities or layering along the falx or tentorium).
DIFFERENTIAL DIAGNOSTIC CONSIDERATIONS FOR IACS
Multiple other lesions must be differentiated for IACs. These include intraaxial cystic tumors (e.g., pilocytic astrocytomas or hemangioblastomas) that typically have a solid and/or enhancing components. Neurocystercercosis can also occur in the arachnoid space (racemose or “grape-like”) but usually comprises multiple cysts. Other differential considerations include a mega cisterna magna and other non-neoplastic cysts (e.g., neuroglial, neurenteric, or porencephalic cysts).[
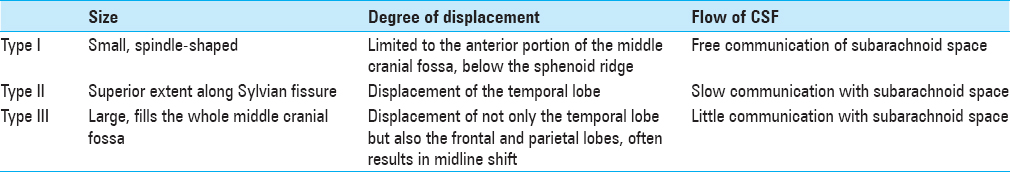
GALASSI CLASSIFICATION OF MIDDLE TEMPORAL FOSSA IACS
MTF IACs account for more than half of all IACs [
CLINICAL PRESENTATION: ASYMPTOMATIC VS. SYMPTOMATIC
Most IAC are asymptomatic and do not require surgery
Most IACs asymptomatic at presentation are incidental and do not require surgery; only 6.8% of large pediatric series show these patients are symptomatic.[
SYMPTOMATIC HEMORRHAGE INTO IAC
Hemorrhage into IACs or rupture into the subdural space leading to subdural CSF hygroma or overt subdural hemorrhage can occur. Hemorrhage rates in the pediatric population are rare (0.3–6%).[
MULTIPLE INDICATIONS FOR IAC SURGERY
In patients with symptomatic IACs, the following variables may contribute to the need for surgery: IAC location, mass effect, impact on CSF flow dynamics (e.g., hydrocephalus), focal neurological deficits, headaches, seizures, and developmental/cognitive deficits [
Suprasellar IACs
Suprasellar IAC can result in compression of the hypothalamic–pituitary axis and thus in endocrinopathies, or of the Foramen of Monroe resulting in obstructive hydrocephalus.
Tectal IACs
Tectal compression from quadrigeminal plate IACs may be responsible for Parinaud's syndrome and/or obstructive hydrocephalus due to compressions of the Aqueduct of Sylvius.
CP angle and middle fossa IACs
Cerebellopontine angle IACs can cause progressive tinnitus, hearing loss, facial palsy, nystagmus, and/or vertigo, whereas MTF IACs can cause temporal/cranial remodeling, deformity, and distortion of orbit with proptosis. The decision for surgical intervention depends on the patient's symptomology.
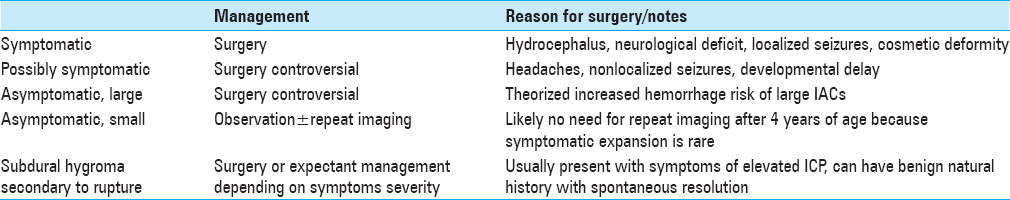
TREATMENT APPROACH
The approach for treating IACs is summarized in
SMALL ASYMPTOMATIC IACS: NO SURGERY
The protocol for patients with small, asymptomatic IACs is the observation with or without repeat imaging. Symptomatic expansion occurs more commonly in children under 4 years or age; these patients warrant serial imaging. For those over the age of 4, symptoms alone may be followed.
SURGICAL INDICATIONS FOR IACS
Surgery is indicated in patients with symptomatic progression associated with hydrocephalus, focal neurological deficits, or localizable seizures. Additionally, large MTF cysts may remodel the overlying frontal, temporal, parietal bones, and/or the orbit resulting in disfigurement. Here, surgery may arrest this process and reduce associated symptoms (e.g., headaches).
DIAGNOSIS AND TREATMENT OF HEADACHES ASSOCIATED WITH IACS
IAC surgery for headaches remains controversial. Kershenovich and colleagues conducted a preoperative trial of acetazolamide to determine who would benefit from surgery; it benefitted 16 of 17 patients (e.g. 13 patients with resolution of symptoms, 3 improved).[
SEIZURES CORRELATED WITH IACS
Some patients with IACs present with seizures. Improved seizure localization may help determine whether IAC surgery is warranted based upon video EEG monitoring, brain electrical source analysis, magnetoencephalography, ictal SPECT imaging, and interictal PET imaging. IAC surgery for poorly localized seizure activity remains controversial.
PROPHYLACTIC IAC SURGERY TO AVERT HEMORRHAGES
Prophylactic surgery has been proposed for very large IACs due to their increased risk of hemorrhage. Unfortunately, there is a lack of data regarding the natural history of these lesions and hemorrhage rates; prophylactic surgery, therefore, remains controversial.
SUBDURAL HYGROMA FROM IAC RUPTURE
IAC rupture can result in symptomatic subdural hygromas. They usually present within days to weeks following a rupture, with progressive elevated ICP (e.g. headache, emesis, CN VI palsy, or papilledema). Traditionally, surgery had been recommended in these patients.[
SURGICAL OPTIONS
There is no consensus regarding the optimal surgery for IACs. Two major surgical options for IACs include cyst shunting (cyst-peritoneal shunt) or cyst wall fenestration into surrounding CSF-filled spaces (cisterns or ventricles). Subdural hygromas or hemorrhages may be associated with IAC, requiring burr hole drainage, with/without fenestration or shunting.
CYST-PERITONEAL SHUNTING
Cyst-peritoneal shunting lowers ICP by CSF diversion, whereas fenestration does not change the CSF volume. Such shunting allows for expansion of the brain parenchyma, but there is no clear benefit unless there is elevated ICP. Nevertheless, this may result in shunt dependence along with its attendant complications. Shunting to the ventricle or subdural space has also been reported in the literature, but data regarding its safety/efficacy are limited.[
CYST FENESTRATION
IAC cyst fenestration avoids shunt-related issues and requires a small craniotomy or, more recently, endoscopic fenestration. The open approach offers the increased ability to control bleeding and perform surgery utilizing a bimanual microsurgical technique versus endoscopy.
BOTH SHUNTING AND CYST FENESTRATION OPTIONS FOR IACS
Fewel et al.[
CASE REPORTS SHORTEN AND CUT MARKEDLY
Case 1
A 14-month-old asymptomatic male, on CT, had a large left supratentorial hemispheric AC (12 × 5 cm, Galassi type 3) [Figure
Case 2
A 4-year-old male presented with vomiting and headache attributed to a Galassi type 2 MR-documented left middle fossa AC. Other comorbidities included global developmental delay (e.g., Trisomy 13 diagnosis), left ventricular noncompaction cardiomyopathy, GERD, a seizure disorder, and tethered cord. A later MRI showed further enlargement of the left middle cranial fossa AC (Galassi type 3) (6 months later) with remodeling of the adjacent sphenoid wing and more mass effect (e.g. increased midline shift) [Figure
Case 3
A 12-year-old male presented with a 3-day history of severe headaches, blurry vision, and vomiting following a helmet-to-helmet collision at football practice. The initial CT and MRI imaging were consistent with a ruptured AC. These studies showed a large right middle cranial fossa AC (Galassi type 3), with remodeling of the adjacent skull, mass effect on the adjacent brain with midline shift medially, and bilateral 4 mm subdural effusions overlying the cerebral hemispheres [Figure
CONCLUSION
There is no Class I evidence regarding the optimal treatment of IACs. We must, therefore, weigh the risks versus benefits of conservative versus surgical treatment of these lesions in symptomatic patients on an individual patient basis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Al-Din A, Williams B. A case of high-pressure intracerebral pouch. J Neurol Neurosurg. 1981. 44: 918-23
2. Al-Holou WN, Terman S, Kilburg C, Garton HJL, Muraszko KM, Maher CO. Prevalence and natural history of arachnoid cysts in adults. J Neurosurg. 2013. 118: 222-31
3. Al-Holou WN, Yew A, Boomsaad Z, Garton HJL, Muraszko KM, Maher CO. Prevalence and natural history of arachnoid cysts in children. J Neurosurg. 2010. 5: 578-85
4. Barkovich A.editors. Diagnostic Imaging: Pediatric Neuroradiology. Thieme, New York: 2014. p.
5. Basaldella L, Orvieto E, Dei Tos AP, Della Barbera M, Valente M, Longatti P. Causes of arachnoid cyst development and expansion. Neurosurg Focus. 2007. 22: E4-
6. Cakir E, Kayhankuzeyli , Sayin OC, Peksoylu B, Karaarslan G. Arachnoid cyst rupture with subdural hygroma: Case report and literature review. Neurocirugia. 2004. 15: 72-5
7. Cress M, Kestle JRW, Holubkov R, Riva-Cambrin J. Risk factors for pediatric arachnoid cyst rupture/hemorrhage. Neurosurgery. 2013. 72: 716-22
8. Di Rocco C, Tamburrini G, Caldarelli M, Velardi F, Santini P. Prolonged ICP monitoring in Sylvian arachnoid cysts. Surg Neurol. 2003. 60: 211-8
9. Fewel ME, Levy ML, McComb G. Surgical treatment of 95 children with 102 intracranial arachnoid cysts. Pediatr Neurosurg. 1996. 25: 165-73
10. Fulkerson DH, Vogel TD, Baker AA, Patel NB, Ackerman LL, Smith JL. Cyst-ventricle stent as primary or salvage treatment for posterior fossa arachnoid cysts. J Neurosurg Pediatr. 2011. 7: 549-56
11. Galassi E, Tognetti F, Gaist G, Fagioli L, Frank F, Frank G. Ct scan and metrizamide CT cisternography in arachnoid cysts of the middle cranial fossa: Classification and pathophysiological aspects. Surg Neurol. 1982. 17: 363-9
12. Garton H, Albright A, Pollack I, Adelson P.editors. The Dandy-Walker complex and arachnoid cysts. In Principles and Practice of Pediatric Neurosurgery. Thieme, New York: 2014. p. 149-57
13. Go KG, Houthoff H-J, Blaauw EH, Havinga P, Hartsuiker J. Arachnoid cysts of the Sylvian fissure. J Neurosurg. 1984. 60: 803-13
14. Gosalakkal JA. Intracranial arachnoid cysts in children: A review of pathogenesis, clinical features, and management. Pediatr Neurol. 2002. 26: 93-8
15. Halani SH, Safain MG, Heilman CB. Arachnoid cyst slit valves: The mechanism for arachnoid cyst enlargement. J Neurosurg Pediatr. 2013. 12: 62-6
16. Helland CA, Aarhus M, Knappskog P, Olsson LK, Lund-Johansen M, Amiry-Moghaddam M. Increased NKCC1 expression in arachnoid cysts supports secretory basis for cyst formation. Exp Neurol. 2010. 224: 424-8
17. Hoffman HJ, Hendrick EB, Humphreys RP, Armstrong EA. Investigation and management of suprasellar arachnoid cysts. J Neurosurg. 1982. 57: 597-602
18. Kershenovich A, Toms S. The acetazolamide challenge: A tool for surgical decision making and predicting surgical outcome in patients with arachnoid cysts. J Neurol Surg Part A Cent Eur Neurosurg. 2016. 78: 33-41
19. Kornienko V, Pronin I.editors. Diagnostic Neuroradiology. Berlin: Springer; 2009. p.
20. Kumagai M, Sakai N, Yamada H, Shinoda J, Nakashima T, Iwama T. Postnatal development and enlargement of primary middle cranial fossa arachnoid cyst recognized on repeat CT scans. Childs Nerv Syst. 1986. 2: 211-5
21. Little JR, Gomez MR, MacCarty CS. Infratentorial arachnoid cysts. J Neurosurg. 1973. 39: 380-86
22. Maher CO, Garton HJ, Al-Holou WN, Trobe JD, Muraszko KM, Jackson EM. Management of subdural hygromas associated with arachnoid cysts. J Neurosurg Pediatr. 2013. 12: 434-43
23. Maiuri F, Gangemi M, Donati PA, Basile D. Chronic hydrocephalus and suprasellar arachnoid cyst presenting with rhinorrhea. Minim Invasive Neurosurg. 1999. 42: 83-5
24. McBride LA, Winston KR, Freeman JE. Cystoventricular shunting of intracranial arachnoid cysts. Pediatr Neurosurg. 2003. 39: 323-9
25. McDonald P, Rutka J. Middle cranial fossa arachnoid cysts that come and go. Report of two cases and review of the literature. Pediatr Neurosurg. 1997. 26: 48-52
26. Osborn AG, Preece MT. Intracranial cysts: Radiologic-Pathologic correlation and imaging approach. Radiology. 2006. 239: 650-64
27. Rao G, Anderson RC, Feldstein NA, Brockmeyer DL. Expansion of arachnoid cysts in children: Report of two cases and review of the literature. J Neurosurg. 2005. 102: 314-7
28. Rengachary SS, Watanabe I. Ultrastructure and pathogenesis of intracranial arachnoid cysts. J Neuropathol Exp Neurol. 1981. 40: 61-83
29. Rengachary SS, Watanabe I, Brackett CE. Pathogenesis of intracranial arachnoid cysts. Surg Neurol. 1978. 9: 139-44
30. Russo N, Domenicucci M, Beccaglia M, Santoro A. Spontaneous reduction of intracranial arachnoid cysts: A complete review. Br J Neurosurg. 2008. 22: 626-9
31. Sandberg DI, McComb JG, Krieger MD. Chemical analysis of fluid obtained from intracranial arachnoid cysts in pediatric patients. J Neurosurg Pediatr. 2005. 103: 427-32
32. Santamarta D, Aguas J, Ferrer E. The natural history of arachnoid cysts: Endoscopic and cine-mode MRI evidence of a slit-valve mechanism. Minim Invasive Neurosurg. 1995. 38: 133-7
33. Seizeur R, Forlodou P, Coustans M, Dam-Hieu P. Spontaneous resolution of arachnoid cysts: Review and features of an unusual case. Acta Neurochir (Wien). 2007. 149: 75-8
34. Starkman SP, Brown TC, Linell EA. Cerebral arachnoid cysts. J Neuropathol Exp Neurol. 1958. 17: 484-500
35. Strahle J, Selzer BJ, Strahle M, Martinez-Sosa M, Muraszko KM, Maher O. Sports participation with arachnoid cysts. 2016. 17: 410-7
36. Wester K. Peculiarities of intracranial arachnoid cysts: Location, sidedness, and sex distribution in 126 consecutive patients. Neurosurgery. 1999. 45: 775-9
37. Wester K, Helland CA. How often do chronic extra-cerebral haematomas occur in patients with intracranial arachnoid cysts?. J Neurol Neurosurg Psychiatry. 2008. 79: 72-5
38. Yildiz H, Erdogan C, Yalcin R, Yazici Z, Hakyemez B, Parlak M. Evaluation of communication between intracranial arachnoid cysts and cisterns with phase-contrast cine MR imaging. Am J Neuroradiol. 2005. 26: 145-51