- Division of Neurosurgery, Kaohsiung Veterans General Hospital, Zuoying, Kaohsiung, Taiwan.
DOI:10.25259/SNI_490_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Chi-Man Yip, Shu-Shong Hsu, Wei-Chuan Liao, Szu-Hao Liu, Yung-Shang Lin, Yu-Hone Hsu, Huang-I Hsu, Yu-Wen Cheng, Yu-Lun Wu. Intracranial solitary fibrous tumor/hemangiopericytoma – A case series. 04-Dec-2020;11:414
How to cite this URL: Chi-Man Yip, Shu-Shong Hsu, Wei-Chuan Liao, Szu-Hao Liu, Yung-Shang Lin, Yu-Hone Hsu, Huang-I Hsu, Yu-Wen Cheng, Yu-Lun Wu. Intracranial solitary fibrous tumor/hemangiopericytoma – A case series. 04-Dec-2020;11:414. Available from: https://surgicalneurologyint.com/surgicalint-articles/10431/
Abstract
Background: Intracranial solitary fibrous tumor/hemangiopericytoma (HPC) is a rare and aggressive tumor. We conducted this retrospective study to investigate the outcome of patients after treatment, the efficacy of postoperative adjuvant radiotherapy, and the factors not conducive to total resection.
Methods: We conducted a retrospective review of the medical records of patients harboring fresh intracranial solitary fibrous tumor/HPC treated from January 2009 to December 2019 in our hospital. We reviewed their clinical presentations, radiologic appearances, tumor size and location, extent of resection, estimate intraoperative blood loss, treatment modalities and results, and duration of follow-up.
Results: There were seven consecutive patients (three males and four females). The ages of the patients at the time of diagnosis ranged from 35 to 77 years (mean: 52.86 years). Five patients (71.43%) got tumor bigger than 5 cm in dimension and only 1 patient (14.29%) underwent gross total tumor resection in the first operation without complication. Five patients (71.43%) underwent postoperative adjuvant radiotherapy. Follow-up period ranged from 4.24 to 123.55 months and the median follow-up period was 91.36 months. Three patients had favorable outcome with Glasgow Outcome Scale (GOS) equal to 4; four patients had unfavorable outcome with GOS equal to 2 or 3. No mortality was happened.
Conclusion: Gross total tumor resection in the initial surgery is very important to achieve a better outcome. Massive intraoperative bleeding and venous sinus or major vessels adjoining are factors not conducive to total resection. Radiotherapy can be administered as adjuvant therapy for cases showing an aggressive phenotype or not treated with gross total resection.
Keywords: Intracranial solitary fibrous tumor/hemangiopericytoma, Massive intraoperative bleeding, Postoperative adjuvant radiotherapy
INTRODUCTION
Before the establishment of 2016 WHO classification of central nervous system (CNS) tumors, solitary fibrous tumor (SFT) and hemangiopericytoma (HPC) represented separate entities. The 2016 WHO classification of CNS tumors combined intracranial SFT and HPC into a single disease entity because of the discovery of NAB2-STAT6 fusion using whole-exome sequencing.[
MATERIALS AND METHODS
We conducted a retrospective review of the medical records of patients having newly diagnosed intracranial SFT/HPC who were treated in our hospital between January 2009 and December 2019. Their age at diagnosis, gender, clinical presentation, radiologic appearances, tumor location, tumor size, extent of resection, estimate blood loss in the first tumor resection surgery, procedure-related complication, WHO grading of the tumor, duration of follow-up, adjuvant therapy, and outcome were reviewed. The extent of resection was deduced from the operative records and the reports of the early postoperative imaging. We used Glasgow Outcome Scale (GOS) to score the outcome of our patients.
RESULTS
There were seven consecutive patients (three males and four females) having newly diagnosed intracranial SFT/HPC treated in our hospital between January 2009 and December 2019. The age of the patients at the time of diagnosis ranged from 35 to 77 years (mean age was 52.86 years). Their initial clinical presentations included focal neurological deficits as well as neuropsychological decline that were related to the tumor location and signs of increased intracranial pressure. The locations of tumors were as follows: convexity = 1; falcine/parasagittal = 2; sphenoidal ridge = 1; cerebellopontine angle = 1; and tentorium cerebelli =2. All tumors had closed attachment or invasion to venous sinus or major vessels. Superior sagittal sinus was involved by the two parasagittal located tumors; transverse sinus was involved by the two tentorium cerebelli located tumors; for the tumor located at the sphenoid ridge, the cavernous sinus, middle cerebral artery, and supraclinoid internal carotid artery were invaded and encased. The cerebellopontine angle located tumor attached the vertebral artery and basilar artery. The convexity located tumor coexisted an unruptured aneurysm of the right middle cerebral artery that had attachment to the tumor [
All of these seven patients had performed brain magnetic resonance imaging (MRI) to study the intracranial lesions before management. On T1-weighted image, the lesion was isointense with cortical gray matter in six cases and hypointense in one case. On T2-weighted image, the lesion was hyperintense in four cases and isointense in three cases. After gadolinium injection, six cases showed heterogeneous enhancement and one case showed homogeneous enhancement [
Figure 1:
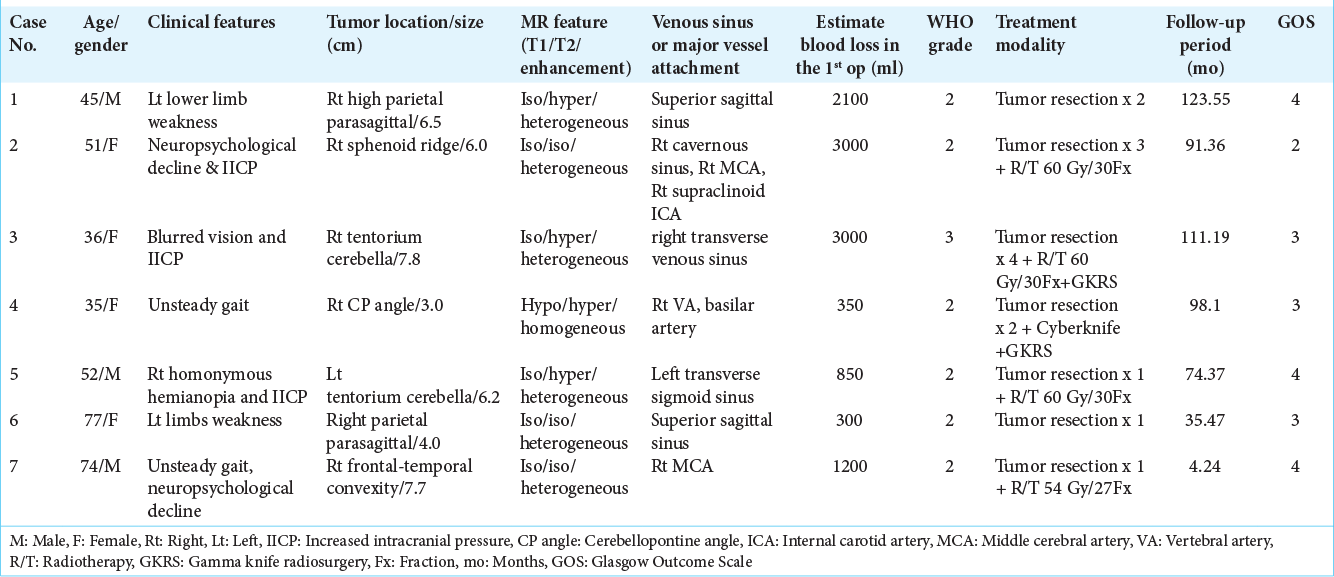
Preoperative brain MRI of case 2. Axial T1-weighted image (a) and axial T2-weighted image (b) showing a large tumor in the right side frontotemporal region, abutting the sphenoid ridge and right cavernous sinus, extension to the right basal ganglion and right side periventricular region with relative isosignal intensity on T1WI and T2WI. Axial T1-weighted image postgadolinium enhancement (c) showing good enhancement. MRA (d) partial encasement of the right middle cerebral artery and right supraclinoid internal carotid artery (black arrows).
Figure 2:
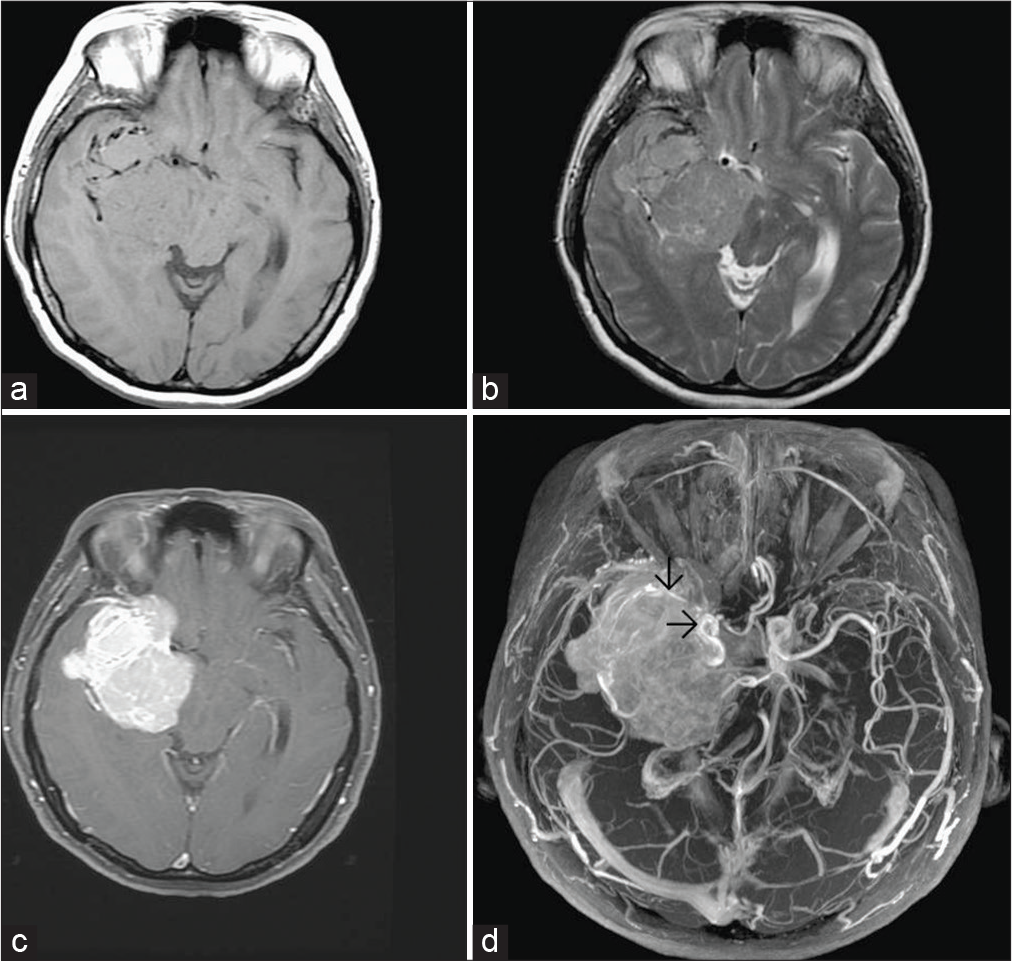
Preoperative brain MRI of case 3. Coronal axial T2-weighted image (a) and coronal T1-weighted image (b) showing a large tumor in right fronto-occipital-parietal region, abutting the posterior cerebral falx and right-sided tentorium and invading right occipital bone, isointense with cortical gray matter on T1-weighted image but hyperintense on T2-weighted image. Coronal T1-weighted image postgadolinium enhancement (c) showing heterogeneous enhancement.
Figure 3:
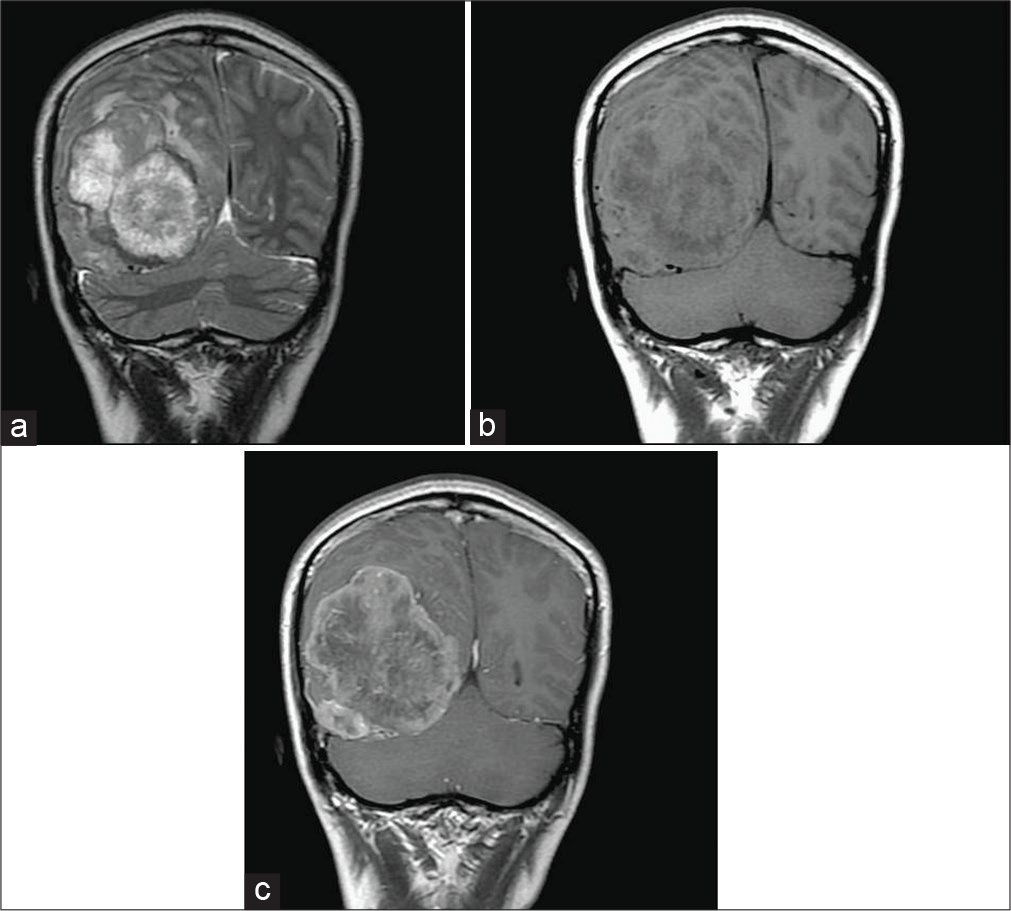
Preoperative brain MRI of case 7. Axial T1-weighted image (a) and axial T2-weighted image (b) showing a large extra-axial well-circumscribed mass lesion in the right frontal-temporal convexity, abutting the skull bone, isointense with cortical gray matter on both T1-weighted and T2-weighted image. Axial T1-weighted image postgadolinium enhancement (c) showing good enhancement. MRA (d) showing a small aneurysm in bifurcation of the right middle cerebral artery (black arrow).
A total of 14 operations were performed on these seven patients for the resection of either primary or residual or recurrent intracranial SFT/HPC. Six patients (85.71%) underwent partial tumor resection in the initial surgery and only 1 patient (14.29%) (case 7) underwent gross total tumor resection in the first operation without complication; the detail of this patient had been reported previously.[
The histopathology of these seven patients was reviewed [
Within these seven patients, 3 patients (42.86%) had favorable outcome with GOS equal to 4; 4 patients (57.14%) had unfavorable outcome with GOS equal to 2 or 3. Two patients (case 2 and case 3) (28.57%) got procedure-related complications including postoperative intracranial bleeding and cerebrospinal fluid leakage, respectively, and needed surgical intervention. No mortality was happened. The follow-up period of these seven patients ranged from 4.24 to 123.55 months and the median follow-up period was 91.36 months [
DISCUSSION
HPC was described in 1942 by Stout and Murray, which accounts less than 1% of all primary CNS tumors.[
Imaging features of HPC include the following: broad-based attachment to the dura, lack calcifications and hyperostosis, multilobulated tumors, heterogeneously hyperdense tumors with focal areas of hypodensity on unenhanced brain computed tomography (CT), heterogeneous or homogeneous enhancement on enhanced brain CT, and isointense with cortical gray matter on T1- and T2-weighted brain MRI and show heterogeneous enhancement on gadolinium-enhanced T1-weighted brain MRI.[
Tihan et al. found that both SFT and HPC had the same microscopic features including spindle to oval cells, “staghorn” vascular pattern, biphasic architecture, and hyalinized vessels. In the field of immunohistochemistry, both tumors showed positive to CD34, Bcl-2, Factor XIIIa, and vimentin, but negative to epithelial membrane antigen and S100 protein.[
Our case number and clinical experience of this unusual tumor are limited. It is hard for us to do any statistical analysis due to limited case number. As compared to other studies,[
CONCLUSION
Gross total tumor resection in the initial surgery is very important to achieve a better outcome. From our limited cases, we notice that massive intraoperative bleeding and venous sinus or major vessels adjoining are factors not conducive to total tumor resection. Radiotherapy including conventional radiotherapy or stereotactic gamma knife radiosurgery can be administered as adjuvant therapy for cases showing an aggressive phenotype or not treated with gross total resection. Improving the handling of intraoperative bleeding including the improvement of surgical strategy and hemostatic agents, further basic research of the nature of this particular tumor, especially the biomolecular field, will help us to advance our treatment.
Declaration of patient consent
Institutional Review Board permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bouvier C, Metellus P, de Paula AM, Vasiljevic A, Jouvet A, Guyotat J. Solitary fibrous tumors and hemangiopericytomas of the meninges: Overlapping pathological features and common prognostic factors suggest the same spectrum of tumors. Brain Pathol. 2015. 22: 511-21
2. Chiechi MV, Smirniotopoulos JG, Mena H. Intracranial hemangiopericytomas: MR and CT features. AJNR Am J Neuroradiol. 1996. 17: 1365-71
3. Clarencon F, Bonneville F, Rousseau A, Galanaud D, Kujas M, Naggara O. Intracranial solitary fibrous tumor: Imaging findings. Eur J Radiol. 2011. 80: 387-94
4. Fountas KN, Kapsalaki E, Kassam K, Feltes CH, Dimopoulos VG, Robinson JS. Management of intracranial meningeal hemangiopericytomas: Outcome and experience. Neurosurg Rev. 2006. 29: 145-53
5. Kim BS, Kim Y, Kong DS, Nam DH, Lee JI, Suh YL. Clinical outcomes of intracranial solitary fibrous tumor and hemangiopericytoma: Analysis according to the 2016 WHO classification of central nervous system tumors. J Neurosurg. 2018. 129: 1384-96
6. Kim YJ, Park JH, Kim YI, Juen SS. Treatment strategy of intracranial hemangiopericytoma. Brain Tumor Res Treat. 2015. 3: 68-74
7. Park BJ, Kim YI, Hong YK, Juen SS, Lee KS, Lee YS. Clinical analysis of intracranial hemangiopericytoma. J Korean Neurosurg Soc. 2013. 54: 309-16
8. Penel N, Amela EY, Decanter G, Robin YM, Marec-Berard P. Solitary fibrous tumors and so-called hemangiopericytoma. Sarcoma. 2012. 2012: 690251
9. Rutkowski MJ, Jian BJ, Bloch O, Chen C, Sughrue ME, Tihan T. Intracranial hemangiopericytoma: Clinical experience and treatment considerations in a modern series of 40 adult patients. Cancer. 2012. 118: 1628-36
10. Schweizer L, Koelsche C, Sahm F, Piro RM, Capper D, Reuss DE. Meningeal hemangiopericytoma and solitary fibrous tumors carry the NAB2-STAT6 fusion and can be diagnosed by nuclear expression of STAT6 protein. Acta Neuropathol. 2013. 125: 651-8
11. Tihan T, Viglione M, Rosenblum MK, Olivi A, Burger PC. Solitary fibrous tumors in the central nervous system. A clinicopathologic review of 18 cases and comparison to meningeal hemangiopericytomas. Arch Pathol Lab Med. 2003. 127: 432-9
12. Yip CM, Lee HP, Fu JH, Hsu SH. Coexistence of intracranial solitary fibrous tumor/hemangiopericytoma and right middle cerebral artery aneurysm. J Surg Case Rep. 2019. 2019: rjz013