- Department of Neurosurgery, Tsukuba Memorial Hospital,
- Department of Neurosurgery, University of Tsukuba, Tsukuba, Ibaraki, Japan.
Correspondence Address:
Eiichi Ishikawa, Department of Neurosurgery, University of Tsukuba, Tsukuba, Ibaraki, Japan.
DOI:10.25259/SNI_79_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kuniyuki Onuma1, Kiyoyuki Yanaka1, Atsushi Tsukada1, Kazuhiro Nakamura1, Yuji Matsumaru2, Eiichi Ishikawa2. Intracranial varix of the transverse-sigmoid dural arteriovenous fistula mimicking a ruptured middle cerebral artery aneurysm: A case report. 25-Mar-2022;13:103
How to cite this URL: Kuniyuki Onuma1, Kiyoyuki Yanaka1, Atsushi Tsukada1, Kazuhiro Nakamura1, Yuji Matsumaru2, Eiichi Ishikawa2. Intracranial varix of the transverse-sigmoid dural arteriovenous fistula mimicking a ruptured middle cerebral artery aneurysm: A case report. 25-Mar-2022;13:103. Available from: https://surgicalneurologyint.com/surgicalint-articles/11487/
Abstract
Background: Hemorrhagic stroke is caused by various vascular abnormalities, such as aneurysms, arteriovenous malformations, and dural arteriovenous fistulas (DAVF). Magnetic resonance angiography (MRA) and three-dimensional computed tomography angiography (3DCTA) are used as efficient initial diagnostic modalities in assessing the etiology of hemorrhagic stroke. We describe the unusual case of a false-positive aneurysm on MRA and 3DCTA.
Case Description: A 65-year-old nonhypertensive woman was brought to our hospital with a sudden onset of headache and left hemiparesis. She also had chemosis in the right eye. CT and magnetic resonance imaging showed an intracerebral hemorrhage in the right temporal lobe. MRA and 3DCTA showed a rounded mass suggestive of an aneurysm arising from the bifurcation of the middle cerebral artery (MCA) and also demonstrated an abnormal tortuous vessel contacting with a rounded mass. Digital subtraction angiography showed a transversesigmoid sinus DAVF with a varix in contact with the MCA bifurcation. Hematoma evacuation and venous drainage disconnection through the right frontotemporal craniotomy were performed.
Conclusion: This case is very instructive and clinicians should keep in mind that detailed neurological and radiological examinations are essential in obtaining an accurate diagnosis, especially if the bleeding source is similar in shape and location to common lesions (such as a cerebral aneurysm).
Keywords: Cerebral aneurysm, Dural arteriovenous fistula, Varix
INTRODUCTION
Hemorrhagic stroke, caused by various vascular abnormalities, including aneurysms, arteriovenous malformations, and dural arteriovenous fistulas (DAVF),[
Digital subtraction angiography (DSA) is the gold standard for diagnosing bleeding sources; however, DSA has the disadvantage of a complication rate reported as about 0.8–2.6%.[
We report a case of intracranial varix of transverse-sigmoid dural arteriovenous fistula mimicking a ruptured cerebral aneurysm. We also present a brief literature review related to this case.
CASE DESCRIPTION
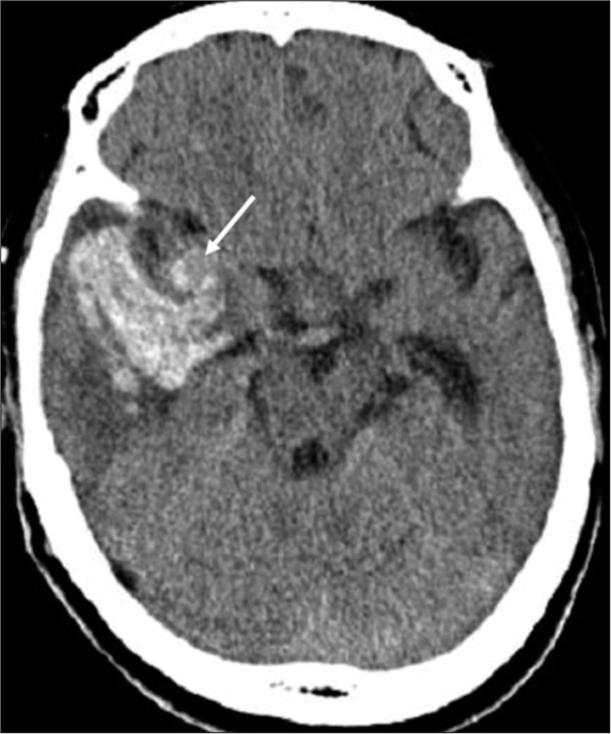
A 65-year-old nonhypertensive woman was brought to our hospital with sudden onset of severe headache. On arrival, she complained of severe headaches and nausea with Glasgow Coma Scale score of 13. Neurological examination exhibited left hemiparesis and conjunctival chemosis of the right eye. Motor power was Grade 3 on the Medical Research Council scale in the left upper and lower extremities. According to her family, the chemosis appeared a few months ago without any trauma. CT and MRI showed an intracerebral hemorrhage (ICH) in the right temporal lobe without subarachnoid hemorrhage and a rounded mass near the right Sylvian fissure [
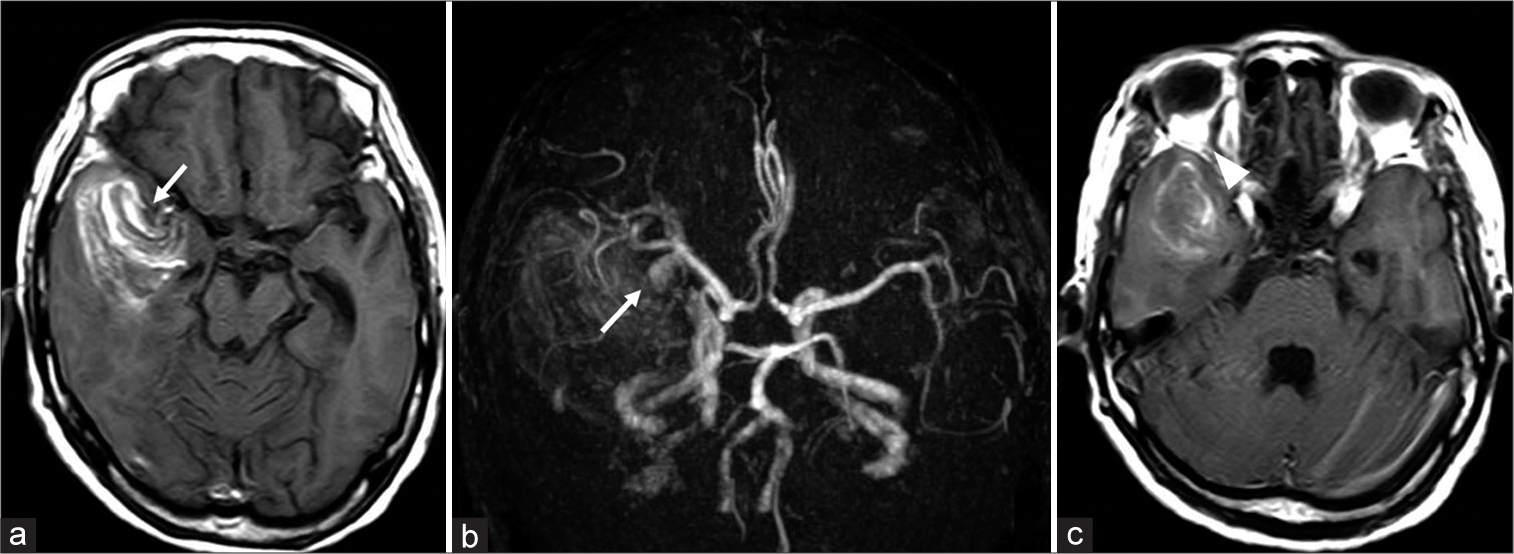
Figure 2:
(a) T1-weighted magnetic resonance imaging showing intracerebral hemorrhage and a rounded mass in contact with the right middle cerebral artery (arrow). (b) MR angiography showing an aneurysmal rounded mass arising from the middle cerebral artery bifurcation (arrow). (c) T1-weighted magnetic resonance imaging showing a dilated right superior orbital vein (arrowhead).
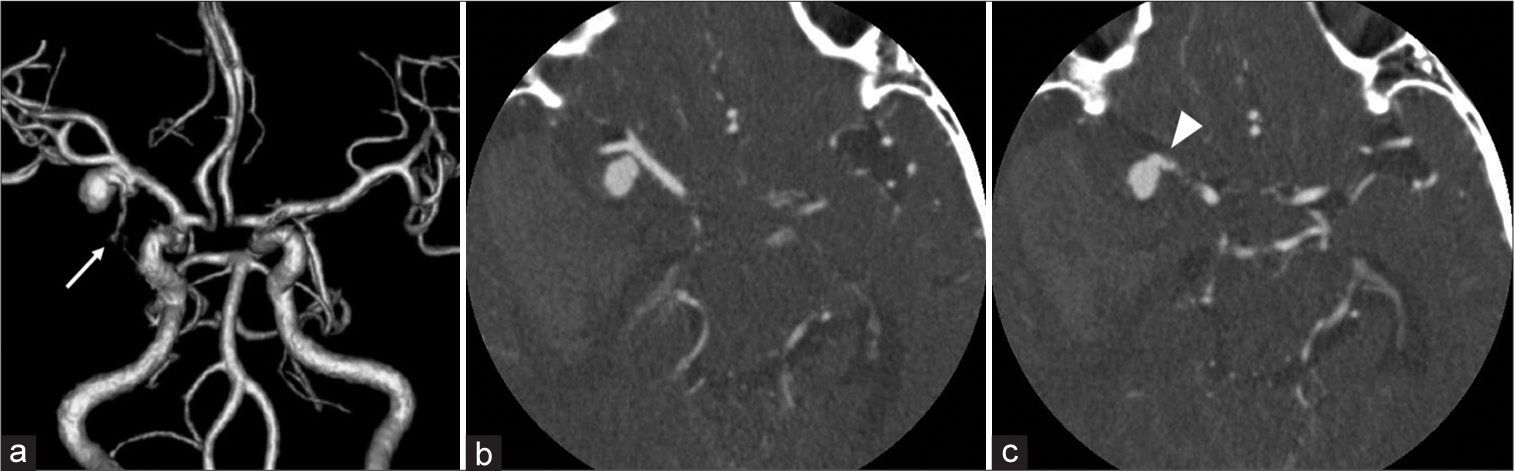
Figure 3:
(a) Three-dimensional computed tomography angiography (3DCTA) showing an aneurysmal rounded mass in contact with the middle cerebral artery bifurcation. (b and c) The aneurysmal rounded mass was separated from the middle cerebral artery on axial source image of CTA and a tortuous vessel arising from the aneurysmal mass (arrow).
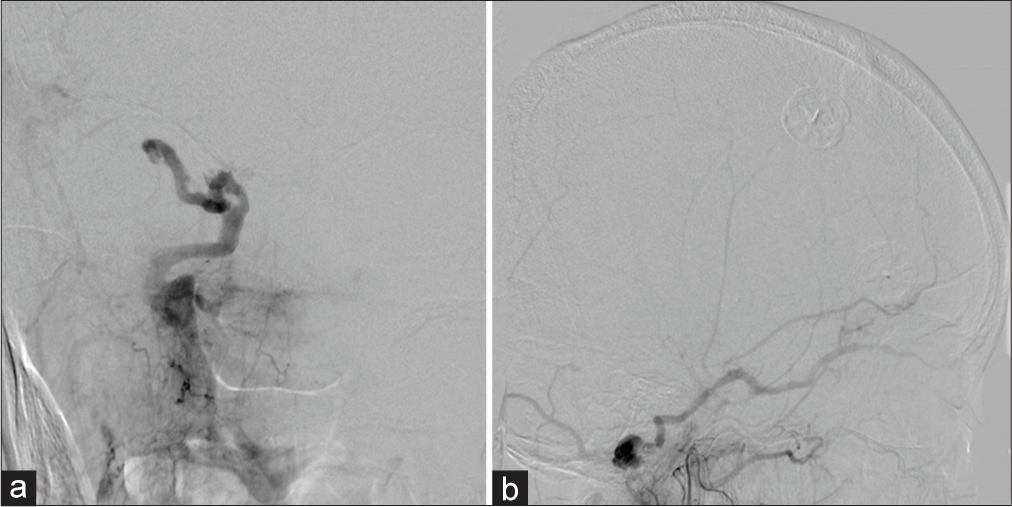
DSA demonstrated a transverse-sigmoid junction DAVF fed by branches of the occipital artery. Early retrograde venous drainage flowed into the straight sinus through the inferior petrosal sinus and internal cerebral veins, forming an intracranial varix. DAVF also regurgitated into the SOV and was thought to be the cause of chemosis. No aneurysm was seen on DSA [
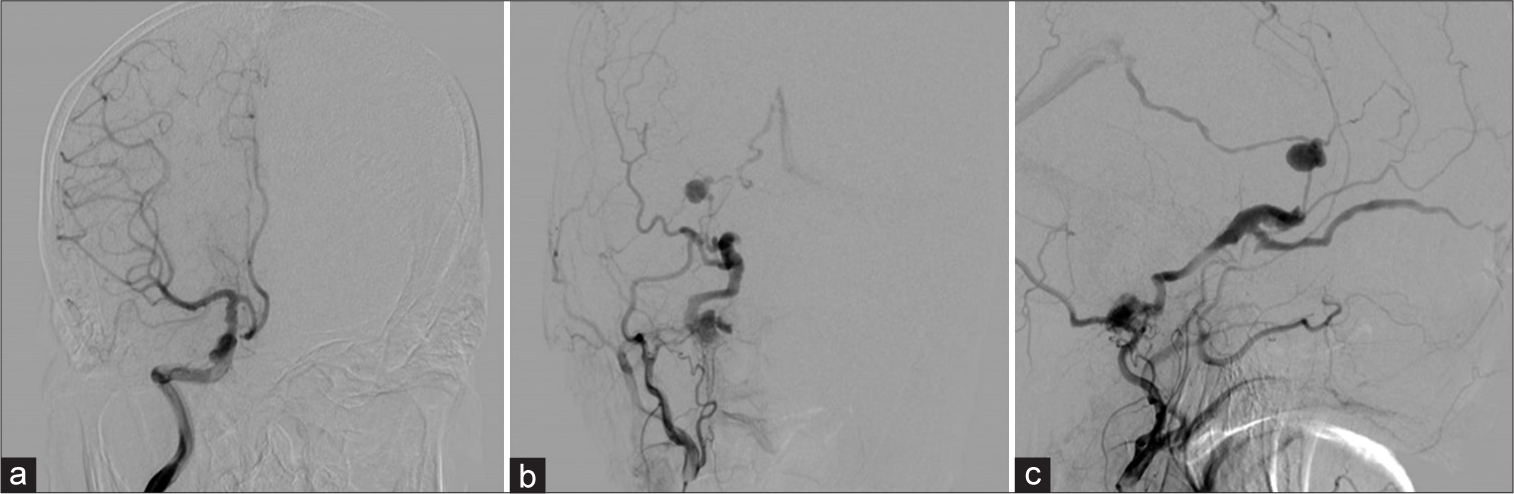
Figure 4:
(a) A right internal carotid angiogram, anteroposterior view, showing no aneurysm. A right external carotid angiogram showing a transverse-sigmoid sinus arteriovenous fistula with the varix, supplied by the occipital artery drained into the straight sinus and right superior ophthalmic vein, (b) anteroposterior view, and (c) lateral view.
A right frontotemporal craniotomy was performed for hematoma evacuation and venous drainage disconnection of the varix to prevent rebleeding and brain herniation in the acute phase. After a dural incision, the Sylvian fissure was opened entirely. Then, the arterialized vein and varix were detected on the surface of the temporal lobe. After occluding the vein temporally using a clip, we verified the absence of varix flow and brain swelling using Doppler ultrasound. The hematoma was also successfully evacuated after ligating the vein and varix. Although we planned endovascular embolization of the residual fistula regurgitated into the SOV [
DISCUSSION
Although DSA is a gold standard for diagnosing the pathophysiology of hemorrhagic stroke, it had been replaced by noninvasive MRI and 3DCTA at the time of initial diagnosis due to its invasiveness. Particularly, in a patient with cerebral aneurysms, 3DCTA and MRA demonstrate high sensitivity and specificity rates that can replace DSA for both detection of this condition and surgical planning.[
There are some reports that other bleeding sources, such as DAVF,[
DAVF sometimes has a retrograde cortical venous drainage with varix and this condition is well known for its high risk of hemorrhagic stroke.[
It is crucial to keep in mind the possibility of various causes for patients with hemorrhagic stroke and carefully interpret data from multiple modalities, including source images, while neurological examination is required for correct pathophysiological diagnosis.
CONCLUSION
This instructive case reminds clinicians to keep in mind that a detailed neurological and radiological examination is essential in obtaining an accurate diagnosis, especially if the bleeding source is similar in shape and location to common lesions (such as a cerebral aneurysm).
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank Dr. Alexander Zaboronok, M.D., Ph.D., Assistant Professor, Department of Neurosurgery, Faculty of Medicine of the University of Tsukuba for professional and English revision, and staff members of the Medical English Communication Center, Faculty of Medicine, the University of Tsukuba, for native English revision.
References
1. Chen Z, Tang W, Liu Z, Li F, Feng H, Zhu G. A dural arteriovenous fistula of the anterior cranial fossa angiographically mimicking an anterior ethmoidal artery aneurysm. J Neuroimaging. 2010. 20: 382-5
2. Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995. 194: 671-80
3. Cohen JE, Gomori JM, Spektor S, Shapiro H, Itshayek E. Symptomatic ethmoidal dural arteriovenous fistula with a draining varix mimicking a ruptured anterior communicating artery aneurysm. Isr Med Assoc J. 2015. 17: 520-1
4. Dawkins AA, Evans AL, Wattam J, Romanowski CA, Connolly DJ, Hodgson TJ. Complications of cerebral angiography: A prospective analysis of 2,924 consecutive procedures. Neuroradiology. 2007. 49: 753-9
5. Earnest FT, Forbes G, Sandok BA, Piepgras DG, Faust RJ, Ilstrup DM. Complications of cerebral angiography: Prospective assessment of risk. AJR Am J Roentgenol. 1984. 142: 247-53
6. Hostettler IC, Seiffge DJ, Werring DJ. Intracerebral hemorrhage: An update on diagnosis and treatment. Expert Rev Neurother. 2019. 19: 679-94
7. Ji YC, Li Y, Hu JX, Zhang HB, Yan PX, Zuo HC. Cerebellar hemangioblastoma mimicking an aneurysm: A case report and literature review. Oncol Lett. 2016. 12: 2622-4
8. Kazemi NJ, Dennien B, Dan NG. Mistaken identity: A case of false positive on CT angiography. J Clin Neurosci. 2002. 9: 464-6
9. Kim JH, Cheong JH, Bak KH, Kim CH, Kim JM. Venous loop mimicking middle cerebral artery bifurcation aneurysm on computed tomographic angiography--case report. Surg Neurol. 2006. 66: 524-6
10. Kwon BJ, Han MH, Kang HS, Chang KH. MR imaging findings of intracranial dural arteriovenous fistulas: relations with venous drainage patterns. AJNR Am J Neuroradiol. 2005. 26: 2500-7
11. Machida T, Hayashi N, Sasaki Y, Aoki S, Shirouzu I, Sasaki Y. Posterior cranial fossa dural arteriovenous malformation with a varix mimicking a thrombosed aneurysm: Case report. Neuroradiology. 1993. 35: 210-1
12. McDonald JS, Kallmes DF, Lanzino G, Cloft HJ. Use of CT Angiography and digital subtraction angiography in patients with ruptured cerebral aneurysm: Evaluation of a large multihospital data base. Am J Neuroradiol. 2013. 34: 1774-7
13. Ogawa T, Okudera T, Miyauchi T, Inugami A, Uemura K, Yasui N. Anterior cranial fossa dural arteriovenous fistula with a varix mimicking an anterior communicating artery aneurysm. Neuroradiology. 1996. 38: 252-3
14. Ohkuma H, Tsurutani H, Suzuki S. Incidence and significance of early aneurysmal rebleeding before neurosurgical or neurological management. Stroke. 2001. 32: 1176-80
15. Patel NP, Robinson TM, Lesley WS, Garrett D, Shan Y, Huang JH. Retromedullary hemangioblastoma mimicking a posterior inferior cerebellar artery aneurysm: Case report and literature review. World Neurosurg. 2019. 122: 165-70
16. Singla N, Aggarwal A, Vyas S, Sanghvi A, Salunke P, Garg R. Glioblastoma multiforme with hemorrhage mimicking an aneurysm: Lessons learnt. Ann Neurosci. 2016. 23: 263-5
17. Tempel ZJ, Johnson SA, Richard PS, Friedlander RM, Rothfus WE, Hamilton RL. Parasellar arachnoid cyst presenting with a nonpupil sparing third nerve palsy mimicking a posterior communicating artery aneurysm in an adult. Surg Neurol Int. 2013. 4: 87
18. Uneda A, Yabuno S, Kanda T, Suzuki K, Hirashita K, Yunoki M. Cavernous angioma presenting with subarachnoid hemorrhage which was diffusely distributed in the basal cisterns and mimicked intracranial aneurysm rupture. Surg Neurol Int. 2017. 8: 202
19. van Asch CJ, Velthuis BK, Greving JP, van Laar PJ, Rinkel GJ, Algra A. External validation of the secondary intracerebral hemorrhage score in The Netherlands. Stroke. 2013. 44: 2904-6
20. van Asch CJ, Velthuis BK, Rinkel GJ, Algra A, de Kort GA, Witkamp TD. Diagnostic yield and accuracy of CT angiography, MR angiography, and digital subtraction angiography for detection of macrovascular causes of intracerebral haemorrhage: Prospective, multicentre cohort study. BMJ. 2015. 351: h5762
21. Wolfe SQ, Manzano G, Langer DJ, Morcos JJ. Cavernous malformation of the oculomotor nerve mimicking a partially thrombosed posterior communicating artery aneurysm: Report of two cases. Neurosurgery. 2011. 69: E470-4
22. Yamada S, Takagi Y, Nozaki K, Kikuta K, Hashimoto N. Risk factors for subsequent hemorrhage in patients with cerebral arteriovenous malformations. J Neurosurg. 2007. 107: 965-72