- Department of Neurosurgery, Rush University Medical Center, Chicago,
- Department of Neurosurgery, NorthShore University HealthSystem, Evanston, Illinois.
Correspondence Address:
Ricky H. Wong
Department of Neurosurgery, NorthShore University HealthSystem, Evanston, Illinois.
DOI:10.25259/SNI_582_2019
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Andrew K. Wong, Ricky H. Wong. Keyhole clipping of a low-lying basilar apex aneurysm without posterior clinoidectomy utilizing endoscopic indocyanine green video angiography. 28-Feb-2020;11:31
How to cite this URL: Andrew K. Wong, Ricky H. Wong. Keyhole clipping of a low-lying basilar apex aneurysm without posterior clinoidectomy utilizing endoscopic indocyanine green video angiography. 28-Feb-2020;11:31. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=9885
Abstract
Background:Basilar apex (BX) aneurysms are surgically challenging due to their anatomic location, need to traverse neurovascular structures, and proximity to multiple perforator arteries. Surgical approaches often require extensive bone resection and neurovascular manipulation. Visualization of low-lying BX aneurysms is typically obscured by the posterior clinoid and upper clivus and poses a unique challenge. Subtemporal or anterolateral approaches with a posterior clinoidectomy are often required to achieve adequate exposure, though these maneuvers can add invasiveness, risk, and morbidity to the procedure. Endoscopes and, more recently, fluoroscopic angiography capable endoscopes offer the possibility of providing improved visualization with less exposure allowing for minimally invasive clipping.
Case Description:We present the case of a 42-year-old female with incidentally found 5 mm middle cerebral artery and 5 mm BX aneurysms. She underwent a minimally invasive supraorbital keyhole craniotomy for the clipping of both aneurysms. While the posterior clinoid obstructed the necessary visualization for the BX aneurysm, use of endoscopy and endoscopic fluoroscopic angiography allowed for safe and successful clipping without the need for a posterior clinoidectomy.
Conclusion:This represents the first reported case of a BX aneurysm clipping through a minimally invasive keyhole craniotomy using endoscopic indocyanine green video angiography. Use of endoscopic indocyanine green angiography, combined with keyhole endoscopic approaches, allows for safe minimally invasive clipping of challenging posterior circulation aneurysms.
Keywords: Basilar apex aneurysm, Endoscopic indocyanine green, Endoscopic surgery, Keyhole craniotomy, Minimally invasive aneurysm clipping
BACKGROUND
Basilar apex (BX) aneurysms are anatomically situated in one of the most challenging areas for a neurosurgeon to access. While the array of endovascular tools used to treat various aneurysms and the proportion of aneurysms treated through such procedures increases, there continues to be a certain subset of patients with BX aneurysms who could benefit from clipping.[
CASE REPORT
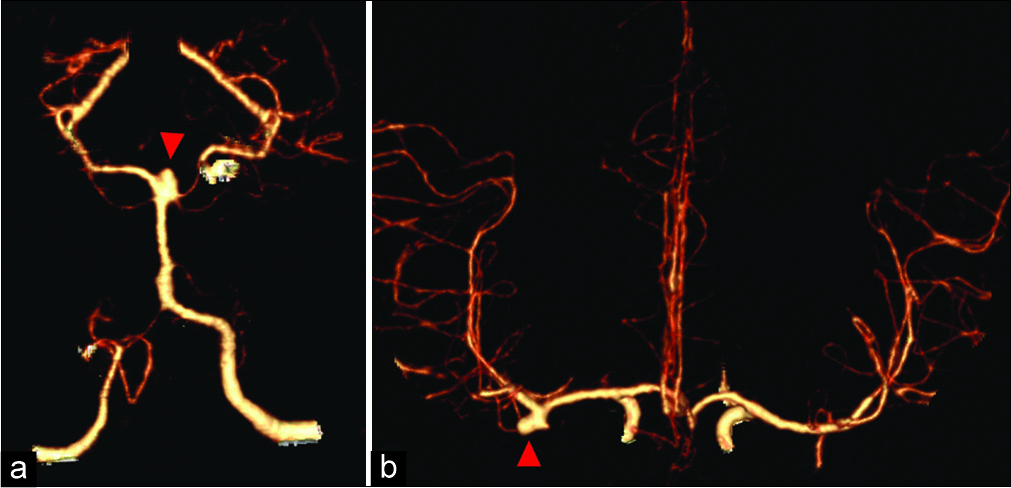
A 42-year-old female presenting with headaches was found to have a 5 mm laterally projecting right middle cerebral artery (MCA) bifurcation aneurysm and a 5 mm BX aneurysm [
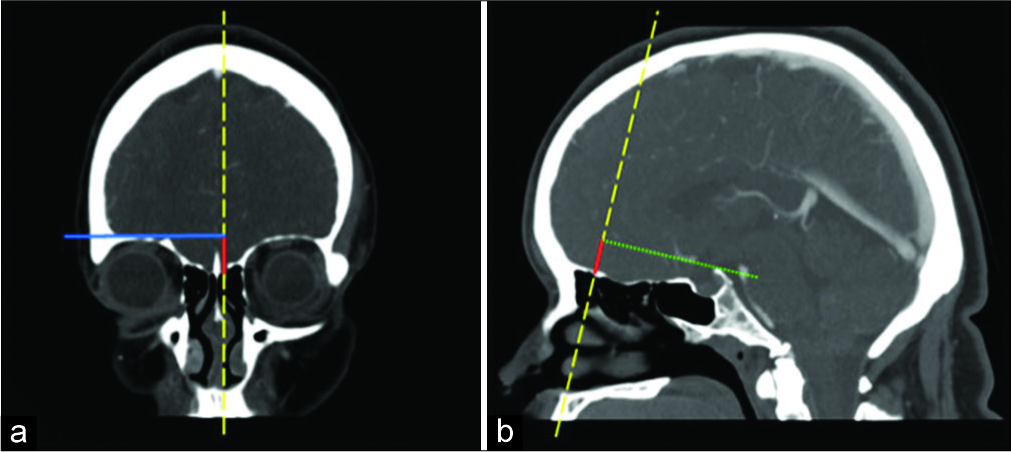
Preoperative computed tomography angiography (CTA) evaluation of the patient’s aneurysms revealed aneurysms amenable for surgical clipping through a supraorbital keyhole approach (SOKA). The MCA bifurcation aneurysm was noted to be at the level of the lesser wing of the sphenoid bone and evaluation of the anatomical accessibility of the BX aneurysm was determined using the orbital roof-dorsum estimation line as previously described.[
Figure 2:
Orbital roof-dorsum estimation line. (a) Orbital height (red line) as measured by the line extending from the anterior skull base along the sagittal scout line (yellow line) to the intersection with the horizontal orthogonal line (blue solid) from the roof of the orbit to the sagittal scout line (b) orbital height line as determined on coronal section translated onto the coronal scout line in the sagittal plane with the orbital roof-dorsum estimation line (green line) extending from the superior point to the top of the posterior clinoid process. In this patient, this line estimates that the most conservative proximal exposure from a supraorbital approach is the neck of the aneurysm.
Operation
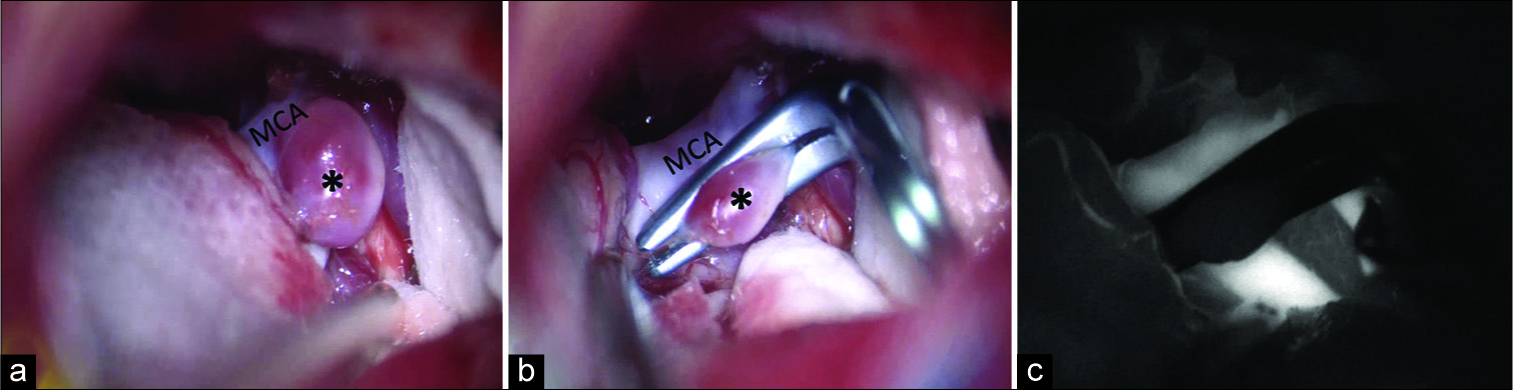
The patient was positioned supine and the head was placed in three-point fixation. A right-sided approximately 5 cm eyebrow incision was made extending from the supraorbital notch to just lateral to the superior temporal line [
Figure 4:
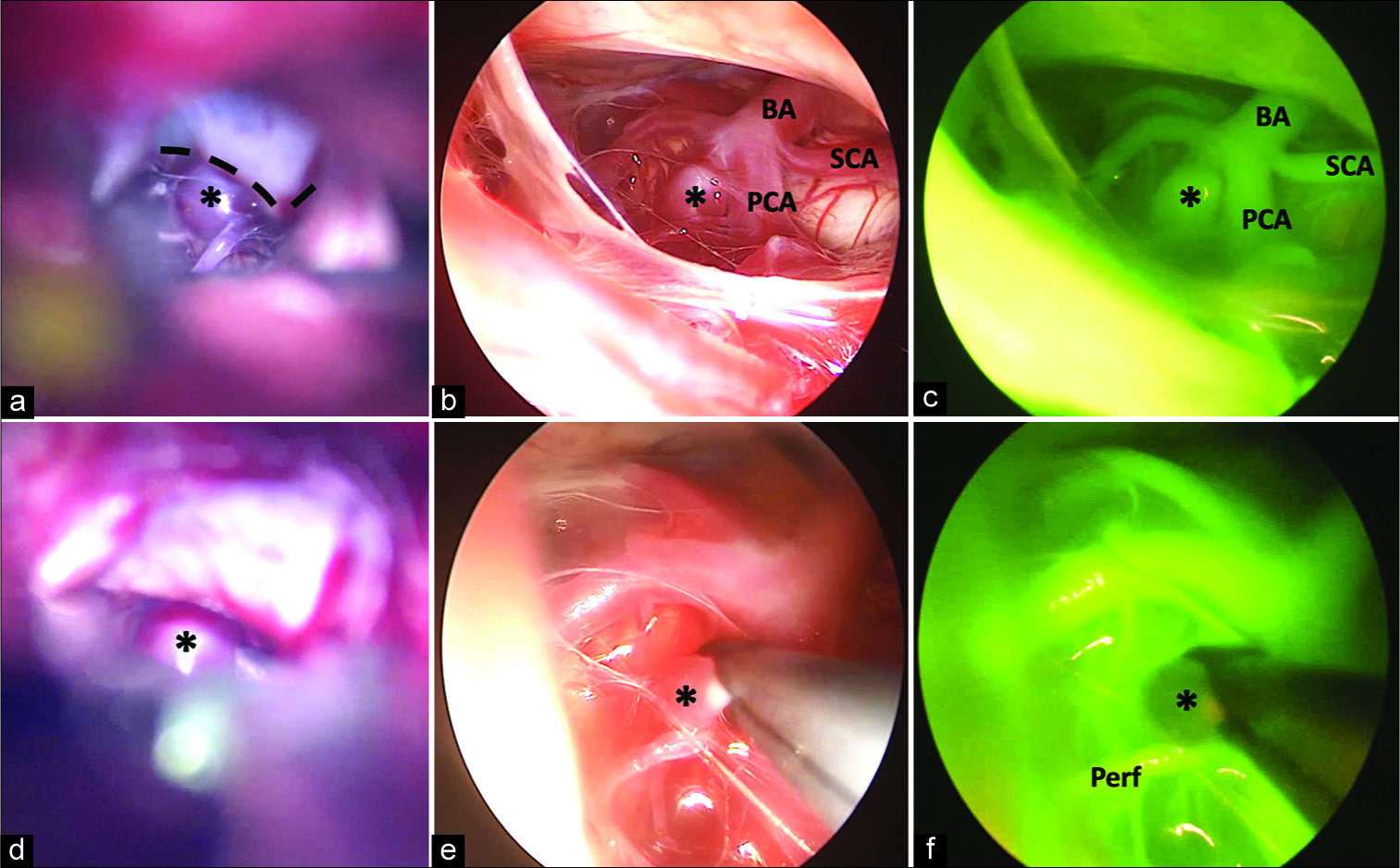
Basilar apex aneurysm (a) microscopic exposure through oculomotor carotid window obstructed by the posterior clinoid (dashed line) (b) endoscopic exposure (c) endoscopic indocyanine green angiography (d) aneurysm clip placement (e) endoscopic view of clip construct (f) endoscopic indocyanine green angiography demonstrating complete aneurysm obliteration with maintenance of perforating arteries. BA: Basilar artery, SCA: Superior cerebellar artery, PCA: Posterior cerebral artery, Perf: Perforator artery, *: Basilar apex aneurysm.
Postoperative course
Postoperative CTA confirmed adequate placement of clips with no residual [
CONCLUSION
Aneurysms of the BX represent some of the most challenging aneurysms for the neurosurgeon. They are situated deep within the intracranial space and bound by neurovascular structures and critical perforators. While there has been a shift toward treating a majority of these aneurysms through endovascular techniques, there remains a subset of patients for whom surgical clipping continues to be the ideal treatment including those with wide-necked or small aneurysms, those who have failed endovascular treatment, and younger patients where a more definitive upfront treatment is desired.[
Critical to the success of any aneurysm surgery is the ability to completely include the aneurysm neck with the surgical clip while maintaining patency of the parent vessel and perforators. Intraoperative strategies to achieve this include electrophysiological monitoring, microDopplers, digital subtraction angiography (DSA), and ICG-VA.[
Classically, approaches to BX aneurysms were through subtemporal, pterional, or orbitozygomatic craniotomies. While the subtemporal approach offers a shorter working distance and a more exposed view of the proximal BA, which can be useful for low lying BX aneurysms, it is significantly limited by the inability to visualize contralateral perforators as well as the need for temporal lobe retraction.[
Improved exposure and visualization of deep-seated structures can alternatively be achieved using endoscopes. By advancing the ocular lens past visually obstructive proximal structures and through the OCW or COW, these gateways become pivot points for the camera rather than bottlenecks.[
In 2013, Bruneau et al. described the first reported use of eICG-VA in aneurysm surgery for a patient with an unruptured anterior communicating artery aneurysm.[
There have been relatively few studies on the use of eICG- VA and most studies to date offer an evaluation of eICG- VA as compared to mICG-VA. As such, surgical exposure was achieved to allow for mICG-VA evaluation and only then was eICG-VA deployed to further scrutinize clip placement.[
In this report, we describe the first minimally invasive keyhole approach for the surgical clipping of a BX aneurysm using eICG-VA. As with any aneurysm clipping, but paramount in BX aneurysms, careful evaluation of preoperative imaging studies as well as a strong understanding of the anatomy is critical to the success of the surgery. Furthermore, knowing the individual strengths and limitations of both the microscope and the endoscope allows you to use them in a complementary fashion to reduce unnecessary exposure and morbidity while maintaining the quality of clip placement. In this case, we were able to estimate the amount of BX exposure we would achieve with a supraorbital approach.[
While endovascular technology continues to evolve, there remains a subset of aneurysms that could benefit from definitive surgical clipping. The full use of surgical technology such as endoscopes and eICG-VA can allow for reduced invasiveness and morbidity while maintaining efficacy in treatment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on:
www.clinicalimagingscience.org
References
1. Bruneau M, Appelboom G, Rynkowski M, Van Cutsem N, Mine B, De Witte O. Endoscope-integrated ICG technology: First application during intracranial aneurysm surgery. Neurosurg Rev. 2013. 36: 77-84
2. Catapano G, Sgulò F, Laleva L, Columbano L, Dallan I, de Notaris M. Multimodal use of indocyanine green endoscopy in neurosurgery: A single-center experience and review of the literature. Neurosurg Rev. 2018. 41: 985-98
3. Cheng CY, Qazi Z, Hallam DK, Ghodke BV, Sekhar LN. Microsurgical clipping of a ruptured basilar apex aneurysm: 3-dimensional operative video. Oper Neurosurg (Hagerstown). 2019. 16: E176-7
4. Chiang VL, Gailloud P, Murphy KJ, Rigamonti D, Tamargo RJ. Routine intraoperative angiography during aneurysm surgery. J Neurosurg. 2002. 96: 988-92
5. Cho WS, Kim JE, Kang HS, Ha EJ, Jung M, Lee C. Dual-channel endoscopic indocyanine green fluorescence angiography for clipping of cerebral aneurysms. World Neurosurg. 2017. 100: 316-24
6. de Oliveira JG, Beck J, Seifert V, Teixeira MJ, Raabe A. Assessment of flow in perforating arteries during intracranial aneurysm surgery using intraoperative near-infrared indocyanine green videoangiography. Neurosurgery. 2008. 62: 1300-10
7. Dogan A, Cetas JS, Anderson GJ, Rekito A, Delashaw JB Jr. Quantitative anterior and posterior clinoidectomy analysis and mobilization of the oculomotor nerve during surgical exposure of the basilar apex using frameless stereotaxis. J Neurol Surg B Skull Base. 2017. 78: 295-300
8. Feindel W, Yamamoto YL, Hodge CP. Intracarotid fluorescein angiography: A new method for examination of the epicerebral circulation in man. Can Med Assoc J. 1967. 96: 1-7
9. Fischer G, Rediker J, Oertel J.editors. Endoscope-versus microscope- integrated near-infrared indocyanine green videoangiography in aneurysm surgery. J Neurosurg. 2018. p. 1-10
10. Greve T, Stoecklein VM, Dorn F, Laskowski S, Thon N, Tonn JC.editors. Introduction of intraoperative neuromonitoring does not necessarily improve overall long-term outcome in elective aneurysm clipping. J Neurosurg. 2019. p. 1-9
11. Hashimoto K, Kinouchi H, Yoshioka H, Kanemaru K, Ogiwara M, Yagi T. Efficacy of endoscopic fluorescein video angiography in aneurysm surgery-novel and innovative assessment of vascular blood flow in the dead angles of the microscope. Oper Neurosurg (Hagerstown). 2017. 13: 471-81
12. Kalavakonda C, Sekhar LN, Ramachandran P, Hechl P. Endoscope-assisted microsurgery for intracranial aneurysms. Neurosurgery. 2002. 51: 1119-26
13. Kim YD, Elhadi AM, Mendes GA, Maramreddy N, Agrawal A, Kalb S. Quantitative study of the opticocarotid and carotid-oculomotor windows for the interpeduncular fossa, before and after internal carotid artery mobilization and posterior communicating division. Neurosurgery. 2015. 11: 162-79
14. Kinouchi H, Futawatari K, Mizoi K, Higashiyama N, Kojima H, Sakamoto T. Endoscope-assisted clipping of a superior hypophyseal artery aneurysm without removal of the anterior clinoid process. Case report. J Neurosurg. 2002. 96: 788-91
15. Klopfenstein JD, Spetzler RF, Kim LJ, Feiz-Erfan I, Han PP, Zabramski JM. Comparison of routine and selective use of intraoperative angiography during aneurysm surgery: A prospective assessment. J Neurosurg. 2004. 100: 230-5
16. Lan Q, Zhang H, Zhu Q, Chen A, Chen Y, Xu L. Keyhole approach for clipping intracranial aneurysm: Comparison of supraorbital and pterional keyhole approach. World Neurosurg. 2017. 102: 350-9
17. Lan Q, Zhu Q, Li G. Microsurgical treatment of posterior cerebral circulation aneurysms via keyhole approaches. World Neurosurg. 2015. 84: 1758-64
18. Li H, Pan R, Wang H, Rong X, Yin Z, Milgrom DP. Clipping versus coiling for ruptured intracranial aneurysms: A systematic review and meta-analysis. Stroke. 2013. 44: 29-37
19. Marlin ES, Ikeda DS, Shaw A, Powers CJ, Sauvageau E. Endovascular treatment of basilar aneurysms. Neurosurg Clin N Am. 2014. 25: 485-95
20. Martin NA, Bentson J, Viñuela F, Hieshima G, Reicher M, Black K. Intraoperative digital subtraction angiography and the surgical treatment of intracranial aneurysms and vascular malformations. J Neurosurg. 1990. 73: 526-33
21. Matsuyama T, Shimomura T, Okumura Y, Sakaki T. Mobilization of the internal carotid artery for basilar artery aneurysm surgery. Technical note. J Neurosurg. 1997. 86: 294-6
22. Menovsky T, Grotenhuis JA, de Vries J, Bartels RH. Endoscope-assisted supraorbital craniotomy for lesions of the interpeduncular fossa. Neurosurgery. 1999. 44: 106-10
23. Mielke D, Malinova V, Rohde V. Comparison of intraoperative microscopic and endoscopic ICG angiography in aneurysm surgery. Neurosurgery. 2014. 10: 418-25
24. Neuloh G, Schramm J. Monitoring of motor evoked potentials compared with somatosensory evoked potentials and microvascular Doppler ultrasonography in cerebral aneurysm surgery. J Neurosurg. 2004. 100: 389-99
25. Nishiyama Y, Kinouchi H, Horikoshi T. Surgery on intracranial aneurysms under simultaneous microscopic and endoscopic monitoring. Clin Neurosurg. 2011. 58: 84-92
26. Nishiyama Y, Kinouchi H, Senbokuya N, Kato T, Kanemaru K, Yoshioka H. Endoscopic indocyanine green video angiography in aneurysm surgery: An innovative method for intraoperative assessment of blood flow in vasculature hidden from microscopic view. J Neurosurg. 2012. 117: 302-8
27. Raabe A, Beck J, Gerlach R, Zimmermann M, Seifert V. Near-infrared indocyanine green video angiography: A new method for intraoperative assessment of vascular flow. Neurosurgery. 2003. 52: 132-9
28. Reisch R, Fischer G, Stadie A, Kockro R, Cesnulis E, Hopf N. The supraorbital endoscopic approach for aneurysms. World Neurosurg. 2014. 82: S130-7
29. Roessler K, Krawagna M, Dörfler A, Buchfelder M, Ganslandt O. Essentials in intraoperative indocyanine green videoangiography assessment for intracranial aneurysm surgery: Conclusions from 295 consecutively clipped aneurysms and review of the literature. Neurosurg Focus. 2014. 36: E7-
30. Salma A, Wang S, Ammirati M. Extradural endoscope-assisted subtemporal posterior clinoidectomy: A cadaver investigation study. Neurosurgery. 2010. 67: ons43-8
31. Stamates MM, Wong AK, Bhansali A, Wong RH. Supraorbital keyhole craniotomy for basilar artery aneurysms: Accounting for the. “Cliff ” effect. Oper Neurosurg (Hagerstown). 2017. 13: 182-7
32. Tang G, Cawley CM, Dion JE, Barrow DL. Intraoperative angiography during aneurysm surgery: A prospective evaluation of efficacy. J Neurosurg. 2002. 96: 993-9
33. Tayebi Meybodi A, Benet A, Rodriguez Rubio R, Yousef S, Mokhtari P, Preul MC. Comparative analysis of orbitozygomatic and subtemporal approaches to the basilar apex: A cadaveric study. World Neurosurg. 2018. 119: e607-16
34. Wainberg RC, da Costa MD, Marchiori M, Soder RB, de Campos Filho JM, Netto HL. Microsurgical clipping of low-riding basilar bifurcation aneurysm. World Neurosurg. 2018. p.
35. Washington CW, Zipfel GJ, Chicoine MR, Derdeyn CP, Rich KM, Moran CJ. Comparing indocyanine green videoangiography to the gold standard of intraoperative digital subtraction angiography used in aneurysm surgery. J Neurosurg. 2013. 118: 420-7
36. Yamada Y, Kato Y, Ishihara K, Ito K, Kaito T, Nouri M. Role of endoscopy in multi-modality monitoring during aneurysm surgery: A single center experience with 175 consecutive unruptured aneurysms. Asian J Neurosurg. 2015. 10: 52-
37. Yoshioka H, Kinouchi H. The roles of endoscope in aneurysmal surgery. Neurol Med Chir (Tokyo). 2015. 55: 469-78
38. Youssef AS, van Loveren HR. Posterior clinoidectomy: Dural tailoring technique and clinical application. Skull Base. 2009. 19: 183-91