- Department of Neurosurgery, Nagoya University, Nagoya,
- Department of Neurosurgery, Aichi Medical University, Nagakute,
- Department of Neurosurgery, Inazawa Municipal Hospital, Inazawa,
- Department of Pathology and Laboratory Medicine, Nagoya University, Nagoya, Japan.
Correspondence Address:
Yusuke Nishimura, Department of Neurosurgery, Nagoya University, Nagoya, Japan.
DOI:10.25259/SNI_453_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Takahiro Oyama1, Yusuke Nishimura1, Yoshitaka Nagashima1, Tomoya Nishii1, Masahito Hara2, Masakazu Takayasu3, Ayako Sakakibara4, Ryuta Saito1. Laminectomy triggers symptomatic growth of spinal schwannoma in a patient with schwannomatosis. 23-Jun-2022;13:261
How to cite this URL: Takahiro Oyama1, Yusuke Nishimura1, Yoshitaka Nagashima1, Tomoya Nishii1, Masahito Hara2, Masakazu Takayasu3, Ayako Sakakibara4, Ryuta Saito1. Laminectomy triggers symptomatic growth of spinal schwannoma in a patient with schwannomatosis. 23-Jun-2022;13:261. Available from: https://surgicalneurologyint.com/surgicalint-articles/11668/
Abstract
Background: Schwannomatosis (SWN) is genetically similar to neurofibromatosis type 2 (NF2) and represents a NF2 gene mutation. Previous studies have shown that these mutations in both neurons and Schwann cells can lead to the development of schwannomas after nerve crush injuries. Here, we reviewed the potential pathoanatomical mechanisms for the development of a trauma-induced spinal schwannomas in a 55-year-old male with SWN.
Case Description: A 49-year-old male had originally undergone a L3–L5 lumbar laminectomy for stenosis; the schwannomas seen on the preoperative magnetic resonance imaging (MRI) were not resected. Now at age 55, he newly presented with low back pain and numbness in the left L5 dermatome, and he was diagnosed with an L4 vertebral level cauda equina tumor on MRI. Following gross-total resection, the histopathological assessment revealed a Ki-67 labeling index 5–10% in hotspots (i.e., slightly higher than the normal range of schwannomas) and a 20% mosaic loss of SMARCB1. Based on these criteria, he was diagnosed as having SWN.
Conclusion: In this patient with SWN, compression/physical trauma to nerves of the cauda equina during the L3–L5 laminectomy 6 years ago likely caused the progression of schwannoma.
Keywords: Laminectomy, Nerve injury, Neurofibromatosis, Schwannomatosis, Spinal schwannoma
INTRODUCTION
Schwannomatosis (SWN) is a rare subtype of neurofibromatosis (NF) and is characterized by multiple schwannomas.[
CASE DESCRIPTION
History
Six years ago, a 49-year-old male had undergone a L3–L5 laminectomy for lumbar canal stenosis; notably, the small intradural schwannomas identified on the preoperative magnetic resonance imaging (MRI) were not removed but helped establish the underlying diagnosis of SWN. Now at age 55, he newly presented with low back pain and numbness in the left L5 dermatome, and he was diagnosed with a L4 vertebral level cauda equina tumor (i.e., either newly developed or progressed) on MRI [
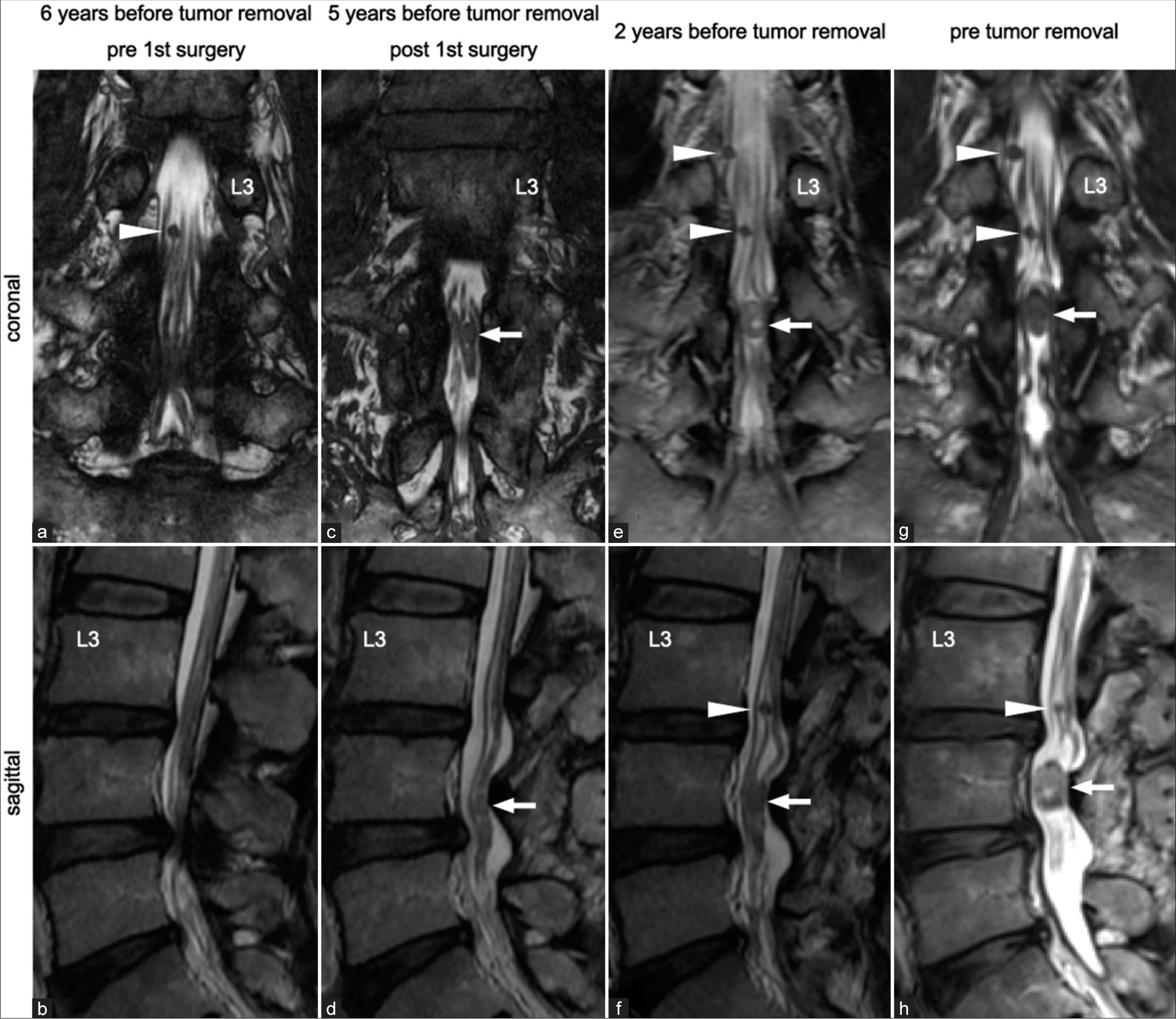
Figure 1:
(a and b) He had originally undergone posterior laminectomy for lumbar canal stenosis at L3–L5 levels at another hospital 6 years before his first visit to us. There seemed no tumors at the L4 vertebral level. Several small tumors (arrowhead) had already existed in the cauda equina before laminectomy. (c and d) He had another tumor pointed out at the L4 vertebral level (arrow) 1 year after surgery. (e-h) Only the newly detected tumor (arrow) had gradually grown in size and had become symptomatic despite other small tumors (arrowhead) remaining the same in size.
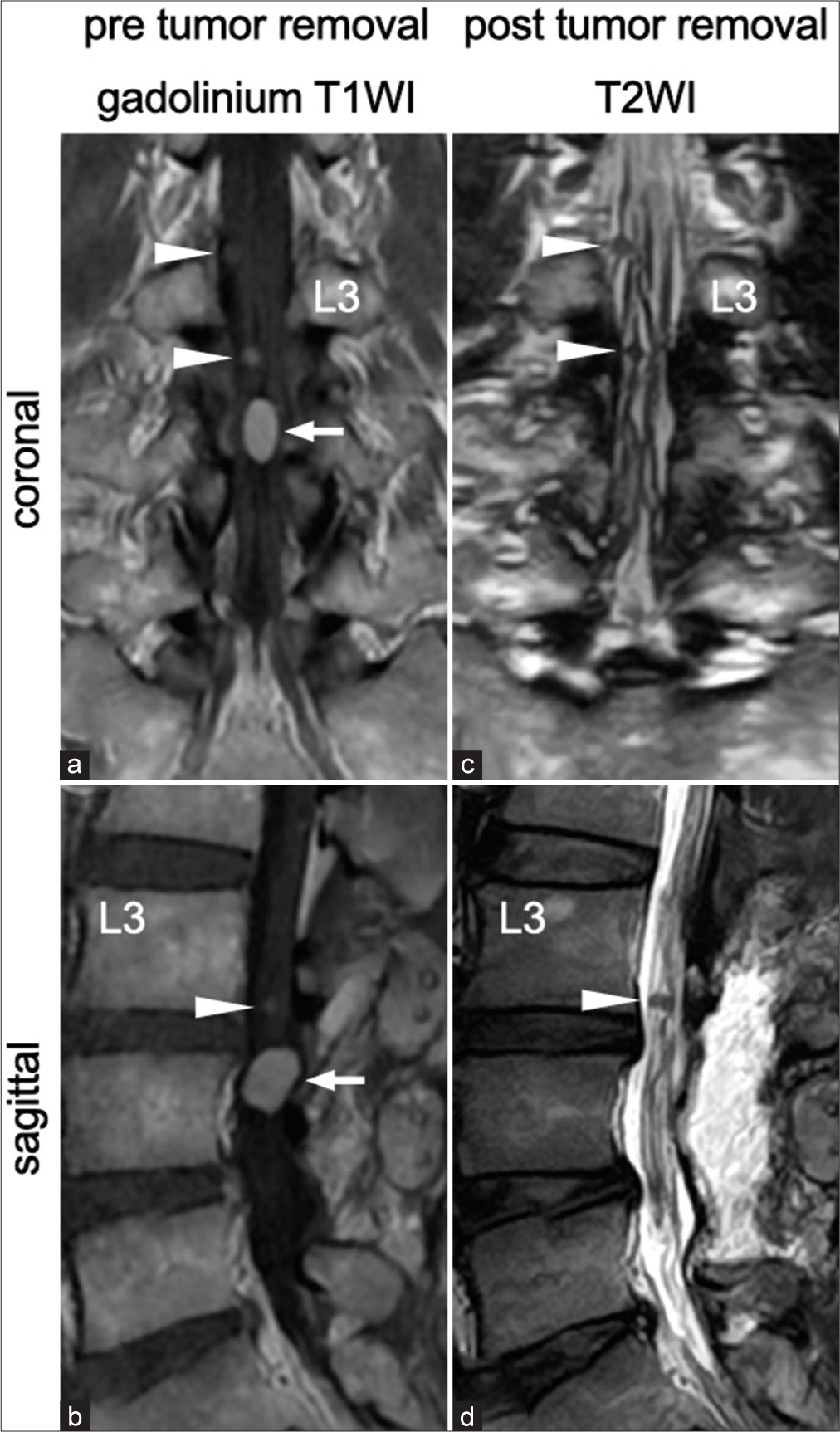
Figure 2:
(a and b) The newly detected tumor (arrow) and several small tumors (arrowhead) were homogeneously enhanced on gadolinium-enhanced T1-weighted images. (c and d) The newly detected tumor was resected completely in the second surgery. The several small tumors (arrowhead) remained the same in size.
Surgical procedure
A revision L3 and L4 laminectomy were performed. With ultrasound guidance, the tumor was visualized within the cauda equina at the L4 vertebral level and was found to be markedly adherent to the surrounding nerve roots. The tumor was totally resected en bloc with careful circumferential tumor dissection using intraoperative neurophysiological monitoring [
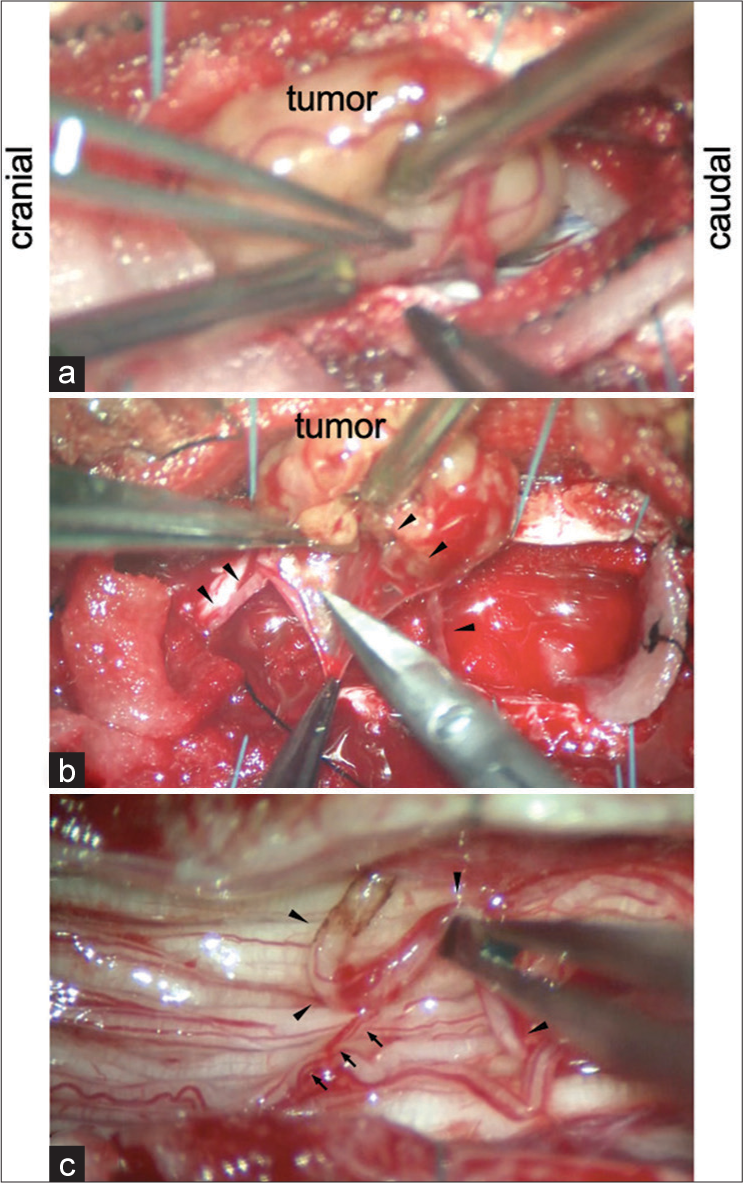
Figure 3:
(a) The tumor adhered to the irrelevant surrounding nerve roots. (b) Careful circumferential and subcapsular dissection allowed us to isolate the tumor from the irrelevant surrounding nerve roots. The nerve of origin was identified (arrowhead). (c) The tumor was resected en bloc by cutting the nerve of origin (arrowhead) following negative confirmation on direct nerve stimulation.
Postoperative course
The hematoxylin and eosin stained sections revealed coexistent hypercellular and hypocellular areas identified as Antoni A and B, respectively, consistent with the diagnosis of a schwannoma [
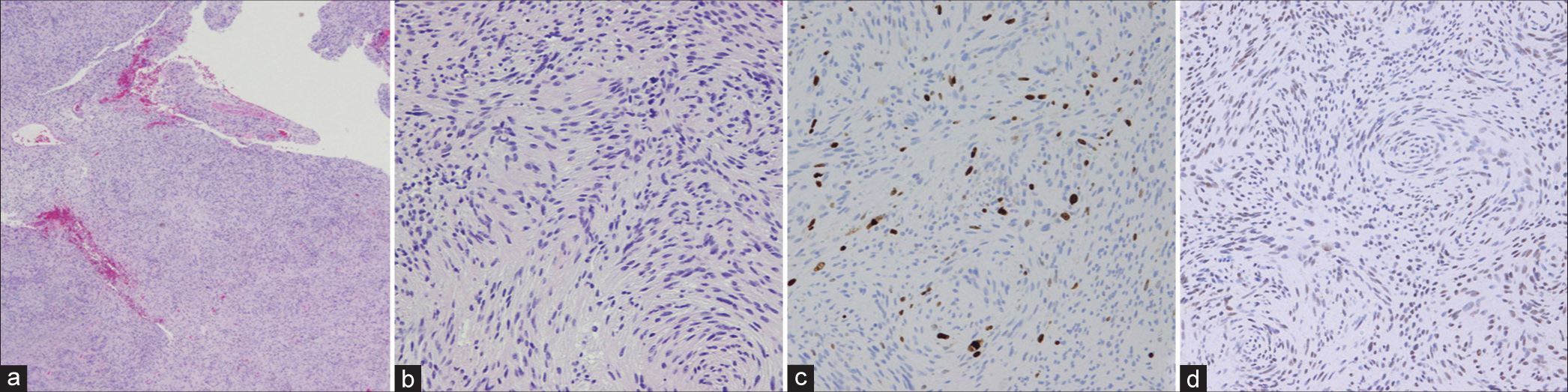
Figure 4:
(a and b) Histopathological analysis of the hematoxylin and eosin stained sections revealed coexistent hypercellular areas and hypocellular areas identified as Antoni A and B, respectively, which are indicative of schwannoma. (c) Ki-67 labeling index was 5–10% in hotspots, which is slightly higher than the normal range of schwannoma. (d) About 20% mosaic loss of SMARCB1 was observed. (a) Objective lens ×5 and (b-d) objective lens ×20.
DISCUSSION
The diagnosis of SWN, as in this patient, was classically based on the identification of two or more nonintradermal schwannomas, one with pathological confirmation without bilateral vestibular schwannoma on high-quality MRI obtained after the age of 30 years and no symptoms of 8th nerve deficits.[
SWN has genetic overlap with NF2
It is difficult to determine the true pathomechanisms of the spinal schwannoma in this patient. However, it is possible that it was due to the underlying diagnosis of SWN and the likely genetic overlap with NF2. Here, the newly detected schwannoma 6 years after the original L3–L5 lumbar laminectomy, although potentially a small lesion on the original preoperative MRI, potentially reflected an elevated Ki-67 labeling index.[
Nerve injuries may promote development of schwannomas
Various types of nerve injuries possibly caused by laminectomy might promote the development of schwannomas.[
CONCLUSION
Compression or physical trauma to nerves occurring during the L3–L5 laminectomy 6 years ago likely caused the development of an L4 vertebral level schwannoma in this patient with SWN.
Declaration of patient consent
The authors certify that all appropriate patient consents have been obtained.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Boyd C, Smith MJ, Kluwe L, Balogh A, MacCollin M, Plotkin SR. Alterations in the SMARCB1 (INI1) tumor suppressor gene in familial schwannomatosis. Clin Genet. 2008. 74: 358-66
2. Caltabiano R, Magro G, Polizzi A, Praticò AD, Ortensi A, D’Orazi V. A mosaic pattern of INI1/SMARCB1 protein expression distinguishes Schwannomatosis and NF2-associated peripheral schwannomas from solitary peripheral schwannomas and NF2-associated vestibular schwannomas. Childs Nerv Syst. 2017. 33: 933-40
3. Evans DG, Bowers NL, Tobi S, Hartley C, Wallace AJ, King AT. Schwannomatosis: a genetic and epidemiological study. J Neurol Neurosurg Psychiatry. 2018. 89: 1215-9
4. Helbing DL, Schulz A, Morrison H. Pathomechanisms in schwannoma development and progression. Oncogene. 2020. 39: 5421-9
5. Koontz NA, Wiens AL, Agarwal A, Hingtgen CM, Emerson RE, Mosier KM. Schwannomatosis: The overlooked neurofibromatosis?. AJR Am J Roentgenol. 2013. 200: W646-53
6. Li P, Zhao F, Zhang J, Wang Z, Wang X, Wang B. Clinical features of spinal schwannomas in 65 patients with schwannomatosis compared with 831 with solitary schwannomas and 102 with neurofibromatosis Type 2: a retrospective study at a single institution. J Neurosurg Spine. 2016. 24: 145-54
7. Mautner VF, Lindenau M, Baser ME. The neuroimaging and clinical spectrum of neurofibromatosis 2. Neurosurgery. 1996. 38: 880-6
8. Meguins LC, Abílio RS, Santos HC, Valsechi LC, Duarte EE, Morais DF. Intradural schwannoma exacerbating the symptoms of degenerative lumbar stenosis: Case report. Arq Bras Neurocir. 2017. 36: 38-42
9. Schellinger KA, Propp JM, Villano JL, McCarthy BJ. Descriptive epidemiology of primary spinal cord tumors. J Neurooncol. 2008. 87: 173-9
10. Schulz A, Büttner R, Hagel C, Baader SL, Kluwe L, Salamon J. The importance of nerve microenvironment for schwannoma development. Acta Neuropathol. 2016. 132: 289-307
11. Sestini R, Bacci C, Provenzano A, Genuardi M, Papi L. Evidence of a four-hit mechanism involving SMARCB1 and NF2 in schwannomatosis-associated schwannomas. Hum Mutat. 2008. 29: 227-31
12. Smith MJ, Wallace AJ, Bowers NL, Rustad CF, Woods CG, Leschziner GD. Frequency of SMARCB1 mutations in familial and sporadic schwannomatosis. Neurogenetics. 2012. 13: 141-5
13. Tamura R. Current understanding of neurofibromatosis Type 1, 2, and schwannomatosis. Int J Mol Sci. 2021. 22: 5850
14. Westhout FD, Mathews M, Pare LS, Armstrong WB, Tully P, Linskey ME. Recognizing schwannomatosis and distinguishing it from neurofibromatosis Type 1 or 2. J Spinal Disord Tech. 2007. 20: 329-32