- Department of Neurosurgery, Yale University School of Medicine, New Haven, Connecticut, United States.
DOI:10.25259/SNI_496_2019
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Christopher S. Hong, Adam J. Kundishora, Aladine A. Elsamadicy, Veronica L. Chiang. Laser interstitial thermal therapy in neuro-oncology applications. 08-Aug-2020;11:231
How to cite this URL: Christopher S. Hong, Adam J. Kundishora, Aladine A. Elsamadicy, Veronica L. Chiang. Laser interstitial thermal therapy in neuro-oncology applications. 08-Aug-2020;11:231. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10197
Abstract
Background: Laser interstitial thermal therapy (LITT) is a minimally invasive surgical treatment for multiple intracranial pathologies that are of growing interest to neurosurgeons and their patients and is emerging as an effective alternative to standard of care open surgery in the neurosurgical armamentarium. This option was initially considered for those patients with medical comorbidities and lesion-specific characteristics that confer excessively high risk for resection through a standard craniotomy approach but indications are changing.
Methods: The PubMed database was searched for studies in the English literature on LITT for the treatment of primary and metastatic brain tumors, meningiomas, as well as for radiation necrosis (RN) in previously irradiated brain tumors.
Results: This review provides an update of the relevant literature regarding application of LITT in neurosurgical oncology for the treatment of de novo and recurrent primary gliomas and brain metastases radiographically regrowing after previous irradiation as recurrent tumor or RN. In addition, this review details the limited experience of LITT with meningiomas and symptomatic peritumoral edema after radiosurgery. The advantages and disadvantages, indications, and comparisons to standard of care treatments such as craniotomy for open surgical resection are discussed for each pathology. Finally, the literature on cost-benefit analyses for LITT are reviewed.
Conclusion: The studies discussed in this review have helped define the role of LITT in neurosurgical oncology and delineate optimal patient selection and tumor characteristics most suitable to this intervention.
Keywords: Brain metastasis, Craniotomy, Glioma, Laser interstitial thermal therapy, Meningioma, Radiation necrosis
INTRODUCTION
With the almost universal availability of Stereotaxis and increasing availability of magnetic resonance imaging (MRI) guidance in surgery, laser interstitial thermal therapy (LITT) has emerged as a popular alternative to standard of care open surgery in the neurosurgical armamentarium, particularly for the management of lesions where access through craniotomy might confer higher morbidity. Laser therapy is based on the delivery of nonionizing radiation as light into targeted tissues which transforms into heat that diffuses out through the tissues causing cellular thermal damage and coagulative necrosis. For LITT in tumors, higher levels of proteins and hemoglobin within the tumor facilitate light absorption compared with water in the surrounding edematous tissue and therefore facilitate preferential heating of the tumor. While thermal ablation has been used for decades to treat tumor, what has revolutionized the field is the ability to monitor tissue temperature change using MR thermometry and then calculate an estimated cell damage zone based on computational estimates of amount of heat delivered overtime to any particular voxel of tissue to control extent of ablation.[
This work reviews the relevant literature regarding application of MRI-guided LITT (MRgLITT) in neurosurgical oncology for the treatment of de novo and recurrent primary gliomas and brain metastases regrowing after previous irradiation as recurrent tumor or radiation necrosis (RN). The limited literature on MRgLITT for meningioma and symptomatic peritumoral edema is also reviewed. The advantages, disadvantages, and considerations for MRgLITT over open surgical resection are presented for both high-grade gliomas (HGGs) [
MRGLITT FOR MALIGNANT TUMORS
Recurrent gliomas
Treatment of HGG remains one of the greatest challenges of neurosurgical oncology. The goal of initial neurosurgical management remains to confer maximal cytoreduction while minimizing morbidity to the patient.[
The first report of MRgLITT in recurrent HGG after prior gross total resection and chemoradiation demonstrated a median local progression-free survival (PFS) of 8 months in a series of 4 patients.[
One of the advantages of MRgLITT is its minimally invasive approach. In a recent publication looking at outcome, 83.2% of patients were able to be discharged home after hospital stay of a mean of only 33.8 h and with a only 1.5% rate of serious adverse events or repeat hospitalization within 30 days of the procedure.[
Case illustration
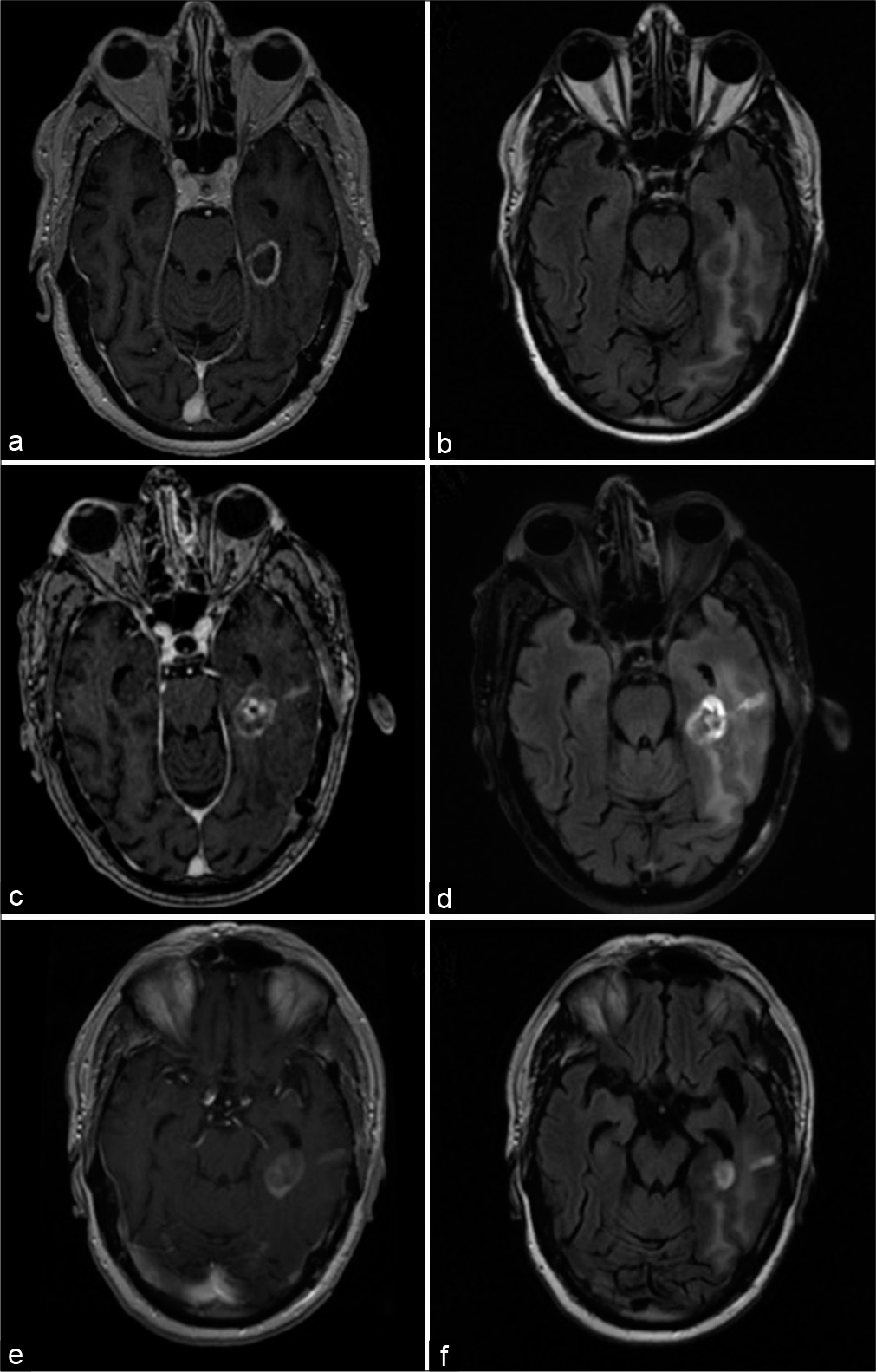
A 65-year-old male was referred to our institution with a prior history of a multifocal glioblastoma with lesions in the left posterior temporoparietal region and a second focus in the left hippocampus, the former of which was resected at an outside institution and subsequently treated with adjuvant temozolomide and radiation. His postoperative course was complicated by speech difficulties secondary to new-onset seizures that were mostly controlled with anticonvulsant therapy, as well as bowel perforation requiring colostomy and deep venous thrombosis, secondary to systemic therapy. At the time of referral, there was radiographic evidence of lesion regrowth at the site of hippocampal disease, concerning for tumor recurrence versus pseudo-progression [
Figure 3:
Clinical imaging for case illustration of recurrent glioblastoma. Preoperative (a) T1-weighted postcontrast and (b) T2- weighted FLAIR magnetic resonance imaging (MRI) demonstrating an enhancing lesion in the left medial temporal region with surrounding edema. Imaging obtained 2 weeks after laser interstitial thermal therapy (LITT) showed a (c) mildly increased size of the enhancing lesion and (d) mild reduction of perilesional edema. An MRI obtained 2 months after LITT demonstrated more definitive (e) reduction in size of the enhancing lesion and (f) further diminishment of edema.
De novo gliomas
Typically, patients with HGG who were either medically unfit for surgery or harbored tumors in surgically inaccessible areas underwent biopsy, followed by standard chemoradiation, resulting in poorer survival outcomes than those undergoing maximal surgical resection.[
In contrast, Kamath et al. in their single institution experienced with MRgLITT for gliomas, reported no significant improvement in outcome after LITT with median PFS and OS of 5.9 months and 11.4 months, respectively, for the treatment of de novo gliomas.[
Based on these limited data, it remains unclear if MRgLITT offers an advantage over biopsy alone in patients with HGG not amenable to open surgery.
Craniotomy versus MRgLITT for gliomas
The ability to compare MRgLITT to standard craniotomy for both recurrent and de novo HGG is limited by a lack of well-designed studies. Barnett et al. performed a systematic review and meta-analysis of outcomes after MRgLITT or craniotomy for gliomas specifically near eloquent areas of the brain.[
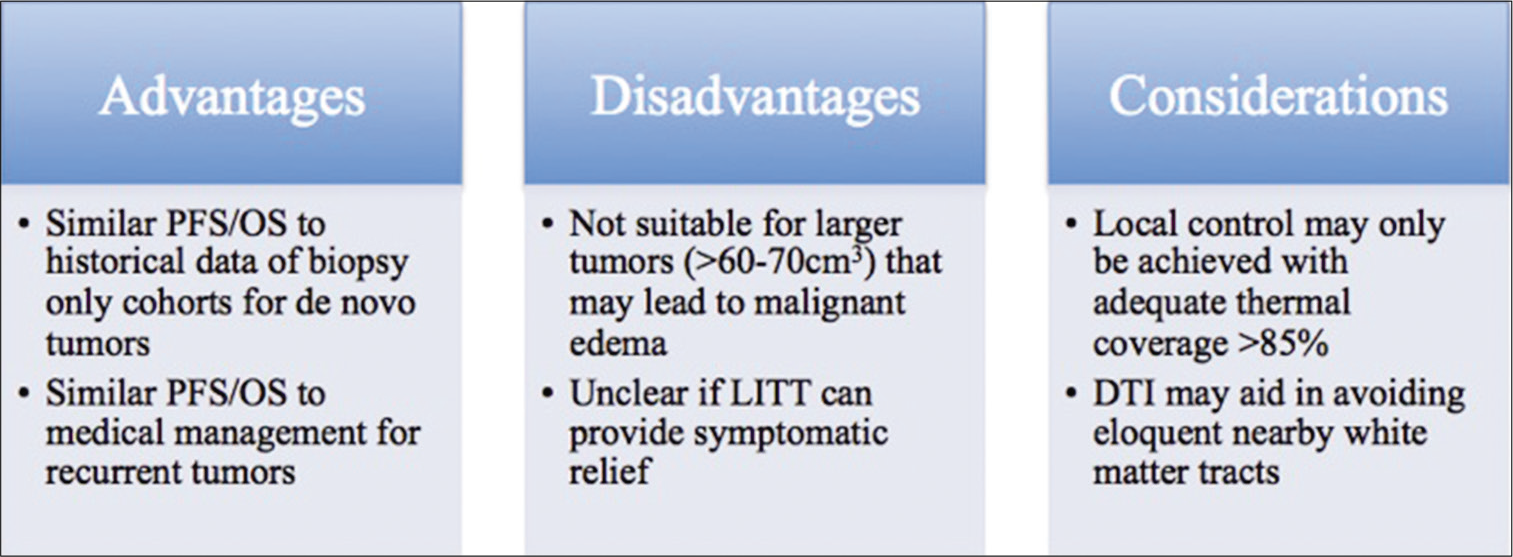
Taken together, MRgLITT may be a viable option for patients with recurrent or de novo HGG who may not be amenable to standard surgical resection and results of LITT may compare favorably to standard medical management with bevacizumab. More appropriate patients may be those of older age, with lower preoperative performance status, and/ or existing medical comorbidities, all of whom may benefit from the minimally invasive nature of LITT with shorter associated hospitalization stays. On the other hand, larger tumors over 60–70cm3 may be unfavorable for MRgLITT due to the potential need for mass decompression, secondary to expected immediate postoperative swelling that occurs in the lesion after LITT. In addition, for tumors near eloquent white matter fiber tracts, use of preoperative DTI may help avoid inadvertent thermal ablation of these tracts and prevent permanent postoperative neurological deficits.
MRGLITT FOR RECURRENT LESIONS AFTER PRIOR SRS TO BRAIN METASTASES
With increasing survival of patients with systemic cancers, local control of brain metastases is becoming more problematic than ever before. In the modern era, brain metastases are managed through a multimodality approach through surgery, adjuvant, and/or upfront radiation therapy with whole brain radiation (WBRT) or stereotactic radiosurgery (SRS) and often novel systemic agents. It is now well recognized that with survival greater than 1 year after brain metastasis treatment, SRS-treated lesions may regrow radiographically due to recurrent tumor, RN, or both. In those who are eligible and willing, surgical management has been an effective salvage treatment. However, in patients unable or unwilling to undergo open surgery, MRgLITT has been proposed as an alternative surgical option.
Recurrent tumors
Carpentier et al. were the first to describe an early pilot clinical trial of 7 patients with 15 total lesions for MRgLITT in regrowing lesions after SRS.[
In 2018, results were reported from laser ablation after stereotactic radiosurgery (LAASR), a multicenter prospective Phase 2 clinical trial of MRgLITT in patients with radiographic progression after SRS for brain metastases,[
RN
In contrast to recurrent tumor, the most compelling evidence for the role and efficacy of MRgLITT has been in the treatment of RN after prior SRS to brain metastases. Early case studies of biopsy-proven RN treated with MRgLITT demonstrated the ability to rapidly wean steroids and improve neurological symptoms.[
Separate from local control, MRgLITT has also proven to be effective for alleviating perilesional edema associated with RN. In their case series of 10 patients with RN, Rammo et al. performed volumetric analyses on T1-weighted postcontrast imaging and found that immediate postoperative lesion volumes increased 220%, further increasing to 430% by 1–2 weeks[
Craniotomy versus MRgLITT for metastatic recurrence and RN
In a recent single-institution retrospective review by Hong et al., 75 patients with lesions regrowing after SRS were compared. Forty-two patients had biopsy-proven tumor and 26 had undergone craniotomy versus 16 how were treated with LITT. The remaining 33 patients had RN – 15 underwent craniotomy versus 18 treated with LITT. Overall, craniotomy was shown to be more effective for providing relief of preoperative neurological symptoms but a larger number of the craniotomy patients with symptoms had lesions >3 cm diameter. Subset analysis of patient with tumors <3 cm in diameter eliminated the symptom relief advantage of craniotomy and further exhibited equivalent 12-month PFS rates (72.2% for LITT vs. 61.1% for craniotomy) and 12-month OS rates (69.0% vs. 69.3%). Further, no difference was found between the two groups with regard to ability to wean off steroids (35% for LITT vs. 47% for craniotomy).
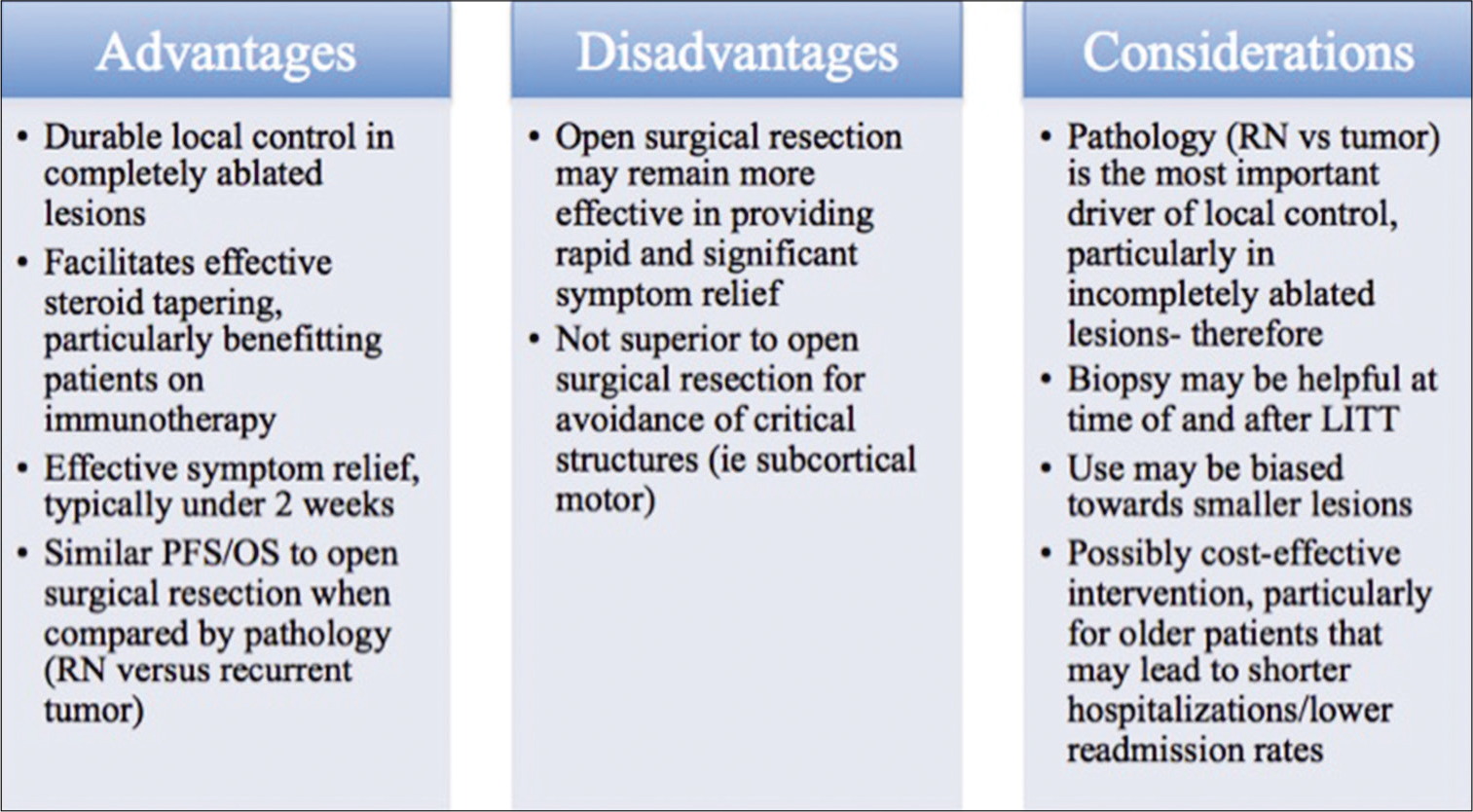
While MRgLITT compares favorably to standard of care craniotomy for the treatment of recurrent brain metastases after prior SRS for lesions <3 cm, what became evident was that pathology of the lesion contributed more significantly to survival outcomes than surgical method. Patients with RN treated with LITT had 12 month PFS of 87.8% and OS of 73.8% not significantly different from patients with RN treated with craniotomy who had 12-month PFS of 86.7% and OS of 93.3%. However, these rates were significantly greater than those seen in patients with tumor, regardless of treatment modality. Patients with tumor treated with LITT had 12-month PFS of 54.7% and OS of 62.5%, statistically similar to patients with tumor treated with craniotomy who had 12-month PFS of 44.4% and OS of 54.3%.[
Taken together, while craniotomy remains better for the management of larger lesions, MRgLITT may be a viable equivalent alternative in patients with lesions with diameters <3 cm, particularly given its effectiveness in the treatment of RN.
Case illustration
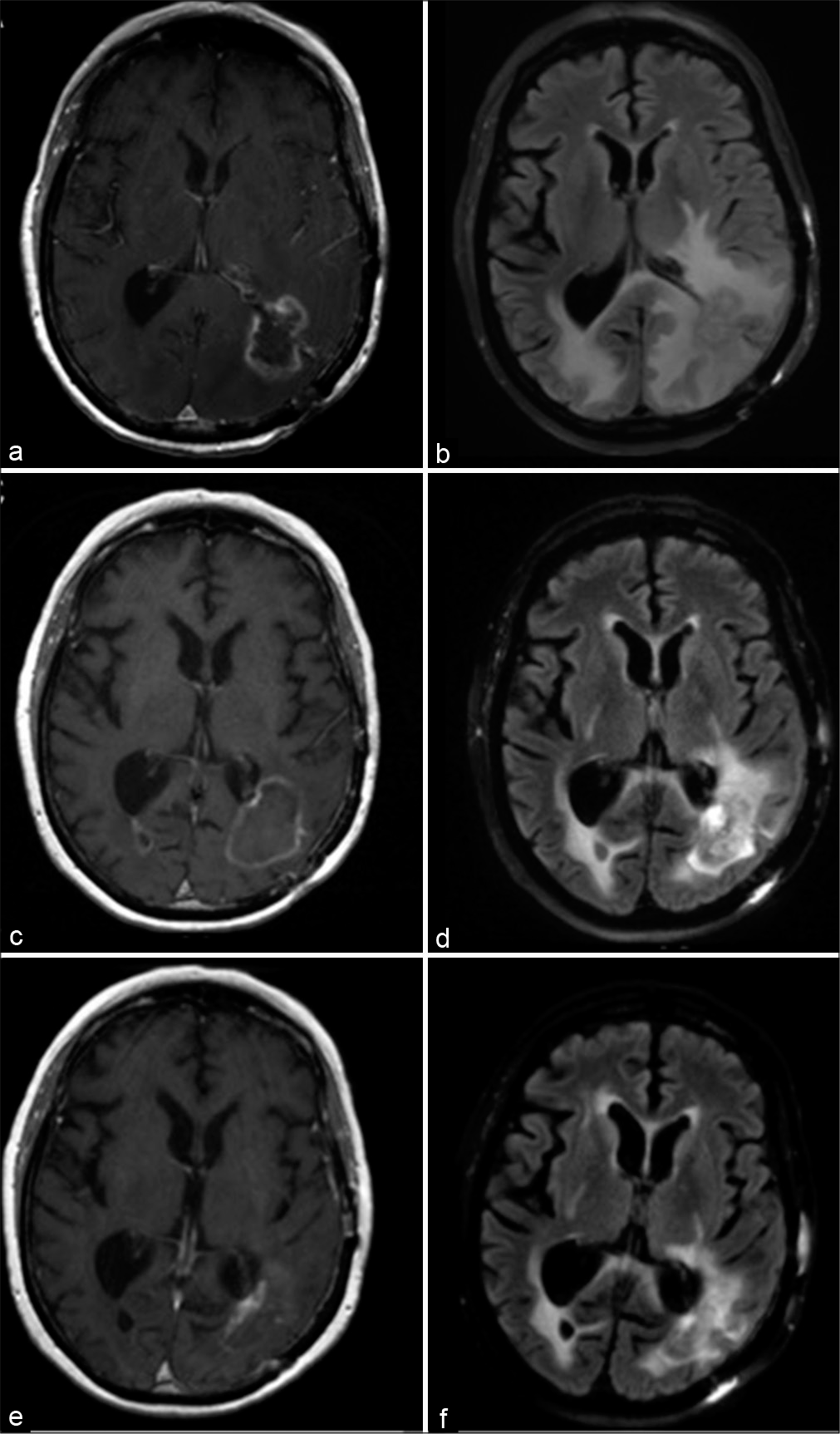
A 60-year-old male with known metastatic nonsmall cell lung cancer underwent surgical resection of a symptomatic left parieto-occipital metastasis followed by consolidative SRS for symptomatic speech difficulty and hemiparesis. Three months later, he underwent additional SRS to a right occipital lesion found on surveillance imaging. Due to lesion regrowth at the site of prior surgery and new dural lesions in the left parietal region, he was treated with whole brain radiation therapy 8 months after initial resection. Nineteen months after surgery, he developed worsening speech comprehension and confusion in the context of regrowing lesions in both parieto-occipital lobes, more radiographically pronounced and symptomatic from the left side, and an inability to be tapered off of steroids [
Figure 4:
Clinical imaging for case illustration of radiation necrosis. Preoperative (a) T1-weighted postcontrast and (b) T2- weighted FLAIR magnetic resonance imaging (MRI) demonstrating an enhancing lesion in the left parieto-occipital region with surrounding edema. Imaging obtained 1 month after laser interstitial thermal therapy (LITT) showed a (c) mildly increased size of the enhancing lesion but (d) reduction of perilesional edema. An MRI obtained 1 year after LITT demonstrated (e) drastic reduction in size of the enhancing lesion and (f) further diminishment of edema.
MRGLITT FOR MENINGIOMA
Meningiomas remain one of the most common primary brain tumors and differ from gliomas and brain metastases by nature of their extra-axial location and typically benign pathology. Surgical resection has remained first-line treatment with radiosurgery reserved for cases of refractory occurrence not amenable to further surgery or higher grade lesions that cannot be totally resected. As such, experience with MRgLITT for meningioma pathology is limited. Ivan et al. reported a case series of five patients treated with MRgLITT at their institution for recurrent radiographically progressing dural-based lesions, comprised three WHO I meningiomas, one WHO III meningioma, as well as a solitary fibrous tumor.[
Symptomatic peritumoral edema (PTE) is a known complication after SRS for meningiomas that may occur in 5–10% of cases.[
While the first-line therapies for meningiomas remain surgical resection through craniotomy and radiotherapy for select cases of residual or higher grade tumors, MRgLITT may be an option for patients who demonstrate lesional regrowth and have exhausted further surgical or radiation options. Based on the limited experience reported in the literature, laser ablation may be more optimal for providing local control in radiographically progressing WHO Grade I tumors, as opposed to higher grades. Likewise, MRgLITT may be efficacious in alleviating symptomatic PTE in cases treated with prior SRS that is not amenable to further surgery. Further studies describing experience with MRgLITT for meningiomas are needed to better determine indications and expected outcomes in these patients.
SOCIOECONOMIC COMPARISON BETWEEN MRGLITT AND CRANIOTOMY
All examinations of novel technology need to take into account cost comparisons with standard of care. Leuthardt et al. analyzed acute care costs for MRgLITT and craniotomy at their institution for both primary and metastatic brain tumors involving difficult to access areas or eloquent brain matter.[
Voigt and Barnett likewise analyzed cost-effectiveness of MRgLITT in 75 patients compared to standard open resection or biopsy only in 890 patients with HGG for whom maximal surgical resection was not feasible.[
The literature on the economic value of MRgLITT remains scarce and further studies may benefit from analysis of specific populations such as the elderly or those with multiple medical comorbidities for whom open surgery may otherwise drive up costs, secondary to longer hospitalization stays, and higher rates of perioperative complications requiring readmission. As evidence for the efficacy of MRgLITT continues to amount in the neurosurgical literature, further studies analyzing its economic value are expected to clarify the circumstances in which MRgLITT may be most cost- effective over standard treatments.
CONCLUSION
MRgLITT continues to grow in popularity as a minimally- invasive alternative to standard of care open surgical resection in neuro-oncology. This review shows that there is growing interest in its use in the treatment of HGG as both upfront therapy and for recurrent tumors, particularly in select patients who may otherwise not be fit for maximal surgical resection. Furthermore, MRgLITT may lead to more favorable outcomes in patients who otherwise are deemed only a surgical candidate for biopsy alone. For patients with recurring lesions after prior radiosurgery to brain metastases, MRgLITT may be an equally efficacious treatment for recurrent tumors as well as for RN after SRS in regard to both patient outcomes and cost-effectiveness. In particular, MRgLITT may confer rapid reductions in perilesional edema and steroid cessation in RN pathology. Preliminary experience with MRgLITT in meningiomas has also suggested a role for its use in recurrent tumors and symptomatic PTE, otherwise not amenable to repeat resection or further radiation. As the use of MRgLITT continues to become more commonplace across institutions, larger studies and clinical trials are expected to determine standardized protocols and indications for MRgLITT in neurosurgical oncology.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Chiang is a consultant for Monteris Medical Inc. (Minnesota, USA) and speaker for BrainLab, Inc. (Munich, Germany).
References
1. Ahluwalia M, Barnett GH, Deng D, Tatter SB, Laxton AW, Mohammadi AM. Laser ablation after stereotactic radiosurgery: A multicenter prospective study in patients with metastatic brain tumors and radiation necrosis. J Neurosurg. 2018. 130: 804-11
2. Ali MA, Carroll KT, Rennert RC, Hamelin T, Chang L, Lemkuil BP. Stereotactic laser ablation as treatment for brain metastases that recur after stereotactic radiosurgery: A multiinstitutional experience. Neurosurg Focus. 2016. 41: E11
3. Barker FG, Chang SM, Gutin PH, Malec MK, McDermott MW, Prados MD. Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery. 1998. 42: 709-20
4. Barnett GH, Voigt JD, Alhuwalia MS. A systematic review and meta-analysis of studies examining the use of brain laser interstitial thermal therapy versus craniotomy for the treatment of high-grade tumors in or near areas of eloquence: An examination of the extent of resection and major complication rates associated with each type of surgery. Stereotact Funct Neurosurg. 2016. 94: 164-73
5. Bastos DC, Rao G, Oliva IC, Loree JM, Fuentes DT, Stafford RJ. Predictors of local control of brain metastasis treated with laser interstitial thermal therapy. Neurosurgery. 2020. 87: 112-22
6. Beechar VB, Prabhu SS, Bastos D, Weinberg JS, Stafford RJ, Fuentes D. Volumetric response of progressing post-SRS lesions treated with laser interstitial thermal therapy. J Neurooncol. 2018. 137: 57-65
7. Carpentier A, Chauvet D, Reina V, Beccaria K, Leclerq D, McNichols RJ. MR-guided laser-induced thermal therapy (LITT) for recurrent glioblastomas. Lasers Surg Med. 2012. 44: 361-8
8. Carpentier A, McNichols RJ, Stafford RJ, Itzcovitz J, Guichard JP, Reizine D. Real-time magnetic resonance-guided laser thermal therapy for focal metastatic brain tumors. Neurosurgery. 2008. 63: ONS21-8
9. Chaunzwa TL, Deng D, Leuthardt EC, Tatter SB, Mohammadi AM, Barnett GH. Laser thermal ablation for metastases failing radiosurgery: A multicentered retrospective study. Neurosurgery. 2018. 82: 56-63
10. De Witt Hamer PC, Robles SG, Zwinderman AH, Duffau H, Berger MS. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: A meta-analysis. J Clin Oncol. 2012. 30: 2559-65
11. Fatima N, Meola A, Pollom E, Chaudhary N, Soltys S, Chang SD. Stereotactic radiosurgery in large intracranial meningiomas: A systematic review. World Neurosurg. 2019. 129: 269-75
12. Hardesty DA, Sanai N. The value of glioma extent of resection in the modern neurosurgical era. Front Neurol. 2012. 3: 140
13. Hawasli AH, Bagade S, Shimony JS, Miller-Thomas M, Leuthardt EC. Magnetic resonance imaging-guided focused laser interstitial thermal therapy for intracranial lesions: Single-institution series. Neurosurgery. 2013. 73: 1007-17
14. Hernandez RN, Carminucci A, Patel P, Hargreaves EL, Danish SF. Magnetic resonance-guided laser-induced thermal therapy for the treatment of progressive enhancing inflammatory reactions following stereotactic radiosurgery, or PEIRs, for metastatic brain disease. Neurosurgery. 2019. 85: 84-90
15. Hong CS, Beckta JM, Kundishora AJ, Elsamadicy AA, Chiang VL. Laser interstitial thermotherapy for treatment of symptomatic peritumoral edema after radiosurgery for meningioma. World Neurosurg. 2020. 136: 295-300
16. Hong CS, Deng D, Vera A, Chiang VL. Laser-interstitial thermal therapy compared to craniotomy for treatment of radiation necrosis or recurrent tumor in brain metastases failing radiosurgery. J Neurooncol. 2019. 142: 309-17
17. Ivan ME, Diaz RJ, Berger MH, Basil GW, Osiason DA, Plate T. Magnetic resonance-guided laser ablation for the treatment of recurrent dural-based lesions: A series of five cases. World Neurosurg. 2017. 98: 162-70
18. Ivan ME, Mohammadi AM, De Deugd N, Reyes J, Rodriguez G, Shah A. Laser ablation of newly diagnosed malignant gliomas: A meta-analysis. Neurosurgery. 2016. 79: S17-23
19. Jethwa PR, Barrese JC, Gowda A, Shetty A, Danish SF. Magnetic resonance thermometry-guided laser-induced thermal therapy for intracranial neoplasms: Initial experience. Neurosurgery. 2012. 71: 133-44
20. Jolesz FA, Talos IF, Schwartz RB, Mamata H, Kacher DF, Hynynen K. Intraoperative magnetic resonance imaging and magnetic resonance imaging-guided therapy for brain tumors. Neuroimaging Clin N Am. 2002. 12: 665-83
21. Kamath AA, Friedman DD, Hacker CD, Smyth MD, Limbrick DD, Kim AH. MRI-guided interstitial laser ablation for intracranial lesions: A large single-institution experience of 133 Cases. Stereotact Funct Neurosurg. 2017. 95: 417-28
22. Kim AH, Tatter S, Rao G, Prabhu S, Chen C, Fecci P. Laser ablation of abnormal neurological tissue using robotic NeuroBlate system (LAANTERN): 12-Month outcomes and quality of life after brain tumor ablation. Neurosurgery. 2020. p. nyaa071
23. Kole AJ, Park HS, Yeboa DN, Rutter CE, Corso CD, Aneja S. Concurrent chemoradiotherapy versus radiotherapy alone for biopsy-only glioblastoma multiforme. Cancer. 2016. 122: 2364-70
24. Leuthardt EC, Voigt J, Kim AH, Sylvester P. A single-center cost analysis of treating primary and metastatic brain cancers with either brain laser interstitial thermal therapy (LITT) or craniotomy. Pharmacoecon Open. 2017. 1: 53-63
25. Lu VM, Jue TR, McDonald KL, Rovin RA. The survival effect of repeat surgery at glioblastoma recurrence and its trend: A systematic review and meta-analysis. World Neurosurg. 2018. 115: 453-459.e3
26. Mohammadi AM, Hawasli AH, Rodriguez A, Schroeder JL, Laxton AW, Elson P. The role of laser interstitial thermal therapy in enhancing progression-free survival of difficult-to-access high-grade gliomas: A multicenter study. Cancer Med. 2014. 3: 971-9
27. Mohammadi AM, Sharma M, Beaumont TL, Juarez KO, Kemeny H, Dechant C. Upfront magnetic resonance imaging-guided stereotactic laser-ablation in newly diagnosed glioblastoma: A multicenter review of survival outcomes compared to a matched cohort of biopsy-only patients. Neurosurgery. 2019. 85: 762-72
28. Patel P, Patel NV, Danish SF. Intracranial MR-guided laser-induced thermal therapy: Single-center experience with the Visualase thermal therapy system. J Neurosurg. 2016. 125: 853-60
29. Rahmathulla G, Recinos PF, Valerio JE, Chao S, Barnett GH. Laser interstitial thermal therapy for focal cerebral radiation necrosis: A case report and literature review. Stereotact Funct Neurosurg. 2012. 90: 192-200
30. Rammo R, Asmaro K, Schultz L, Scarpace L, Siddiqui S, Walbert T. The safety of magnetic resonance imaging-guided laser interstitial thermal therapy for cerebral radiation necrosis. J Neurooncol. 2018. 138: 609-17
31. Rammo R, Scarpace L, Nagaraja T, Lee I. MR-guided laser interstitial thermal therapy in the treatment of recurrent intracranial meningiomas. Lasers Surg Med. 2019. 51: 245-50
32. Rao MS, Hargreaves EL, Khan AJ, Haffty BG, Danish SF. Magnetic resonance-guided laser ablation improves local control for postradiosurgery recurrence and/or radiation necrosis. Neurosurgery. 2014. 74: 658-67
33. Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011. 115: 3-8
34. Seo Y, Kim DG, Kim JW, Han JH, Chung HT, Paek SH. Long-term outcomes after gamma knife radiosurgery for benign meningioma: A single institution’s experience with 424 Patients. Neurosurgery. 2018. 83: 1040-9
35. Shao J, Radakovich NR, Grabowski M, Borghei-Razavi H, Knusel K, Joshi KC. Lessons learned in using laser interstitial thermal therapy for treatment of brain tumors: A case series of 238 Patients from a single institution. World Neurosurg. 2020. 139: e345-4
36. Sloan AE, Ahluwalia MS, Valerio-Pascua J, Manjila S, Torchia MG, Jones SE. Results of the NeuroBlate system first-in-humans Phase I clinical trial for recurrent glioblastoma: Clinical article. J Neurosurg. 2013. 118: 1202-19
37. Smith CJ, Myers CS, Chapple KM, Smith KA. Long-term follow-up of 25 Cases of biopsy-proven radiation necrosis or post-radiation treatment effect treated with magnetic resonance-guided laser interstitial thermal therapy. Neurosurgery. 2016. 79: S59-72
38. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005. 352: 987-96
39. Torres-Reveron J, Tomasiewicz HC, Shetty A, Amankulor NM, Chiang VL. Stereotactic laser induced thermotherapy (LITT): A novel treatment for brain lesions regrowing after radiosurgery. J Neurooncol. 2013. 113: 495-503
40. Voigt JD, Barnett G. The value of using a brain laser interstitial thermal therapy (LITT) system in patients presenting with high grade gliomas where maximal safe resection may not be feasible. Cost Eff Resour Alloc. 2016. 14: 6
41. Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017. 377: 1954-63