- Department of Neurosurgery, Neurological Institute of Thailand, Bangkok, Thailand
- Department of Neuroradiology, Neurological Institute of Thailand, Bangkok, Thailand
- Department of Radiology, Bumrungrad International Hospital, Bangkok, Thailand.
Correspondence Address:
Prasert Iampreechakul, Department of Neurosurgery, Neurological Institute of Thailand, 312 Rachawithi Road, Khwaeng Thung Phaya Thai, Bangkok, Thailand.
DOI:10.25259/SNI_45_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Prasert Iampreechakul1, Korrapakc Wangtanaphat1, Yodkhwan Wattanasen2, Sunisa Hangsapruek2, Punjama Lertbutsayanukul2, Somkiet Siriwimonmas3. Long-term surveillance in an infant with spontaneous obliteration of pial arteriovenous malformation and large intranidal aneurysm: A unique case observation. 21-Jun-2024;15:206
How to cite this URL: Prasert Iampreechakul1, Korrapakc Wangtanaphat1, Yodkhwan Wattanasen2, Sunisa Hangsapruek2, Punjama Lertbutsayanukul2, Somkiet Siriwimonmas3. Long-term surveillance in an infant with spontaneous obliteration of pial arteriovenous malformation and large intranidal aneurysm: A unique case observation. 21-Jun-2024;15:206. Available from: https://surgicalneurologyint.com/surgicalint-articles/12956/
Abstract
Background: Spontaneous obliteration of untreated cerebral arteriovenous malformations (AVMs) is rare, occurring in
Case Description: The authors reported a remarkably rare instance of spontaneous thrombosis in a pial AVM accompanied by a large intranidal aneurysm in a 10-month-old infant, initially presenting with a nocturnal seizure. Diagnostic imaging revealed a ruptured intranidal aneurysm causing acute hemorrhage in the left anterior interhemispheric subdural space, extending into adjacent areas. Further, magnetic resonance imaging (MRI) and magnetic resonance angiography delineated the AVM in the left superior frontal gyrus, associated with a thrombosed aneurysm and surrounding edema. Cerebral angiography confirmed the AVM’s origin from the left anterior cerebral artery, displaying early venous drainage and small, indirect feeders not amenable to endovascular treatment. Over time, serial imaging showed the aneurysm’s transition from partial to complete thrombosis. Subsequent MRIs and angiographic assessments up to age 10 confirmed complete resolution of the AVM and aneurysm, with focal hyperemia persisted until age 16, when recurrent AVM was identified.
Conclusion: We document a rare spontaneous regression of a pial AVM with an intranidal aneurysm influenced by specific vascular factors. Despite this, spontaneous thrombosis should not replace vigilant long-term monitoring in pediatric neurovascular care.
Keywords: Pial Arteriovenous Malformation, Pediatric Neurovascular, Spontaneous Thrombosis, Focal hyperemia, Recurrent brain arteriovenous malformations, Intranidal aneurysm
INTRODUCTION
Brain arteriovenous malformations (AVMs) represent a complex and challenging neurovascular disorder, particularly in pediatric populations, and create a unique set of clinical challenges. These malformations are abnormal tangles of blood vessels and are characterized by direct connections between arteries and veins in the brain without an intervening capillary bed.[
The pial AVMs are a rare neurovascular anomaly in children under 2 years of age. These AVMs begin to resemble adult counterparts in their physiology, type, and architecture when children reach the age of 5–7 years.[
In 2019, we reported on an extremely rare case of a 10-month-old infant who experienced complete spontaneous thrombosis of a pial AVM associated with a large intranidal aneurysm.[
The present study extends the observations of this patient from now to 16 years of age, providing a unique long-term perspective on the natural history and neurodevelopmental outcomes of post spontaneous thrombosis of an AVM in a pediatric patient. This extended follow-up has allowed us to observe not only the stability of the thrombosis over a prolonged period but also to evaluate the long-term neurological and developmental impact of such rare spontaneous events in pediatric neurovascular disorders. Our findings contribute to a deeper understanding of the long-term clinical course and radiological evolution of pediatric AVMs, particularly those that undergo spontaneous resolution, and highlight the necessity for ongoing surveillance in such cases.
CASE DESCRIPTION
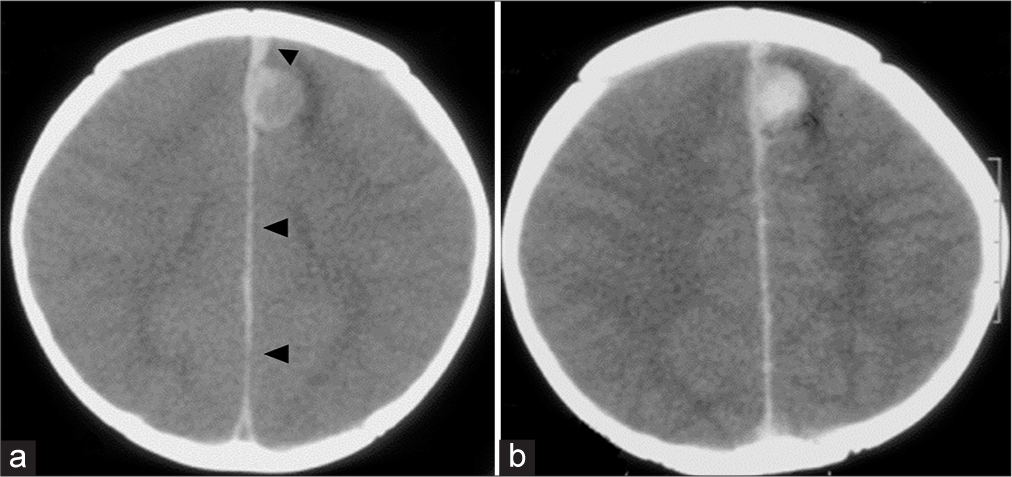
A 10-month-old female infant was admitted to a community hospital after experiencing a nocturnal seizure observed by her mother. The seizure involved screaming, eyes rolling up, and generalized convulsions lasting for 2 min, with no preceding fever or trauma. Born at term with a birth weight of 3080 g, her developmental history had been unremarkable. Initial diagnostic imaging through a computed tomography scan revealed a hyperdense, approximately 15 mm mass with surrounding edema in the left superior frontal gyrus and an adjacent acute thin subdural hematoma (SDH) [
Figure 1:
At the presentation. Axial views of cranial computed tomography scan (a) without and (b) with contrast show an enhancing hyperdense round mass, approximately 15 mm in size, with surrounding edema at the medial aspect of the left superior frontal gyrus. There was a small hyperdense lesion (black arrowheads) located at the left side of the anterior interhemispheric fissure adjacent to the medial part of the mass, extending posteriorly along the interhemispheric fissure, representing acute interhemispheric subdural hemorrhage.
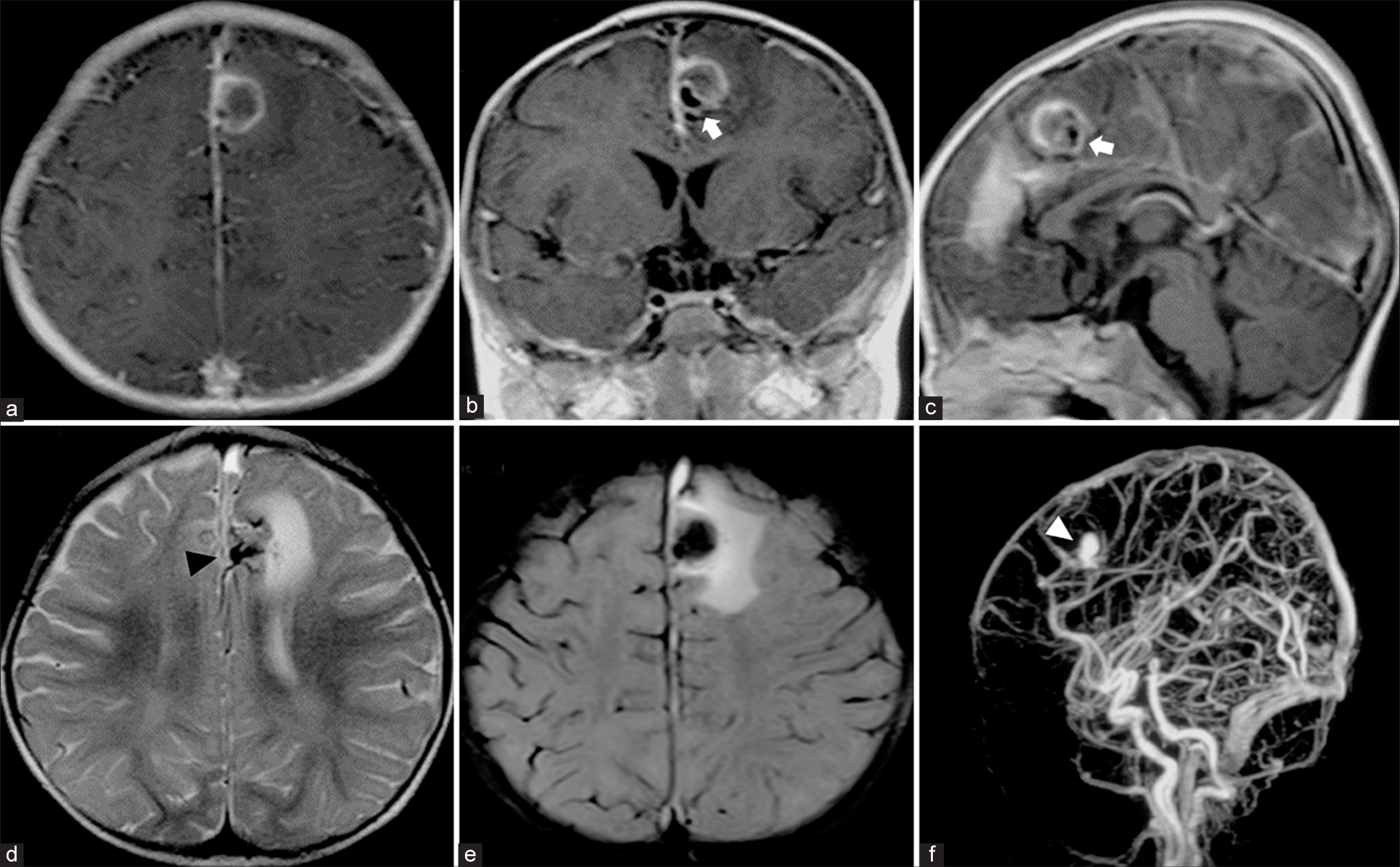
A follow-up magnetic resonance imaging (MRI) obtained a week post symptom onset showed the SDH extending along the posterior interhemispheric fissure, posterior falx, bilateral tentorial cerebelli, and over both hemispheric convexities, more pronounced on the left side. Abnormal flow voids suggestive of a pial AVM were noted in the left superior frontal region. The left anterior interhemispheric SDH adjoined the medial side of the large round mass, indicating a probable source of hemorrhage from a ruptured aneurysm. T1-weighted gadolinium-enhanced MRI delineated the mass as a large, partially thrombosed aneurysm with eccentric signal voids and peripheral enhancement. T2-weighted MRI highlighted the progression of perianeurysmal edema in the same region. Magnetic resonance angiography (MRA) confirmed the pial AVM with a large intranidal aneurysm [
Figure 2:
Magnetic resonance imaging (MRI) of the brain obtained 1 week after symptom onset. (a) Axial, (b) coronal, and (c) sagittal T1-weighted contrast-enhanced MRI show peripheral rim enhancing round mass with an eccentrically located signal void (white arrows), probably indicating a large partially thrombosed aneurysm. (d and e) Axial T2-weighted MRI reveals a tangle of abnormal flow voids (black arrowhead), probably representing pial arteriovenous malformations associated with a large aneurysm with the progression of perianeurysmal edema at the medial side of the left superior frontal region. (f) Sagittal maximum intensity projection image from 3D time-of-flight magnetic resonance angiography confirms the pial arteriovenous malformation with a large intranidal aneurysm (white arrowhead).
After a 3-week hospitalization, the infant was clinically stable and referred to our institute for potential endovascular treatment. Physical examination revealed mild developmental delay, closed anterior fontanel, and normal vital signs, with measurements of 44 cm head circumference, 7.5 kg weight, and 70 cm length. Neurological examination showed no signs of meningeal irritation, papilledema, or other neurological deficits.
One month after symptom onset, a follow-up MRI indicated complete resolution of the SDH and a laminated appearance in the partially thrombosed aneurysm, with persistent perianeurysmal edema [
Figure 3:
Magnetic resonance imaging (MRI) of the brain obtained 1 month after symptom onset. Sagittal T1-weighted MRI (a) with and (b) without contrast show heterogeneous signal intensity with eccentrically rim enhancing lesion, probably representing a laminated appearance of partially thrombosed aneurysm. (c) Axial and (d) coronal T2-weighted MRI reveals heterogeneous signal intensity in a large aneurysm surrounded by edema of the left superior frontal gyrus.
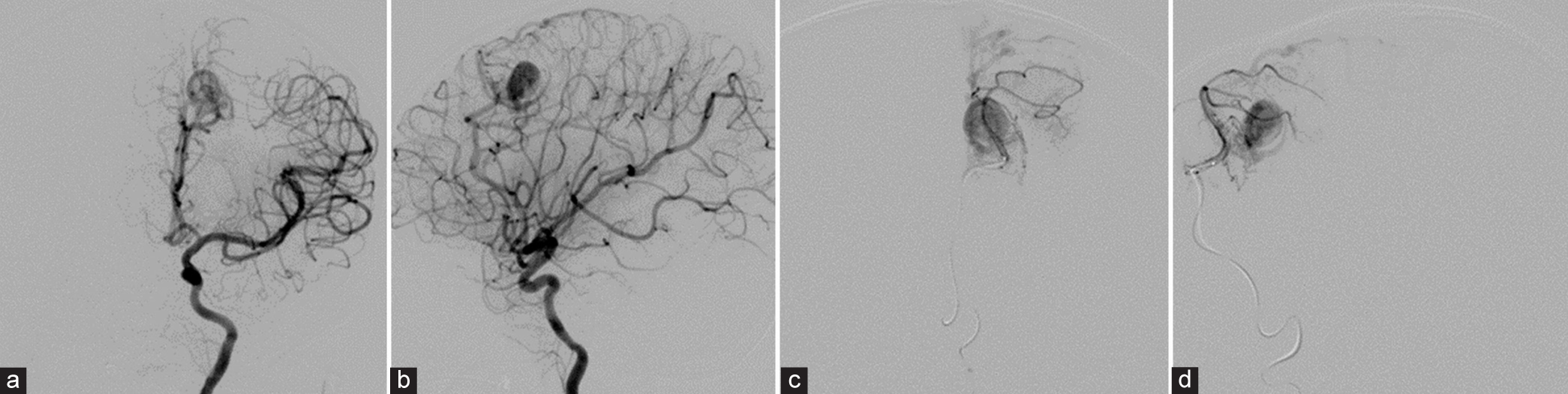
Figure 4:
(a) Anteroposterior (AP) and (b) lateral views of the left internal carotid artery injection show a small pial arteriovenous malformation supplied by the left middle internal frontal branches of the left anterior cerebral artery (ACA) with early venous drainage into medial frontal veins and forward to superior sagittal sinus. A large aneurysm was located within the small nidus. (c) AP and (d) lateral views of the left ACA injection with microcatheter clearly demonstrate a small nidus, multiple indirect small feeders, large intranidal aneurysm, and early draining veins.
Figure 5:
Magnetic resonance imaging (MRI) of the brain obtained 7 months after symptom onset. Sagittal T1-weighted MRI (a) with and (b) without contrast demonstrate a significant reduction in the size of a large partially thrombosed aneurysm. (c) Axial and (d) coronal T2-weighted MRI revealed a reduction in aneurysm size and complete resolution of perianeurysmal edema at the left superior frontal gyrus.
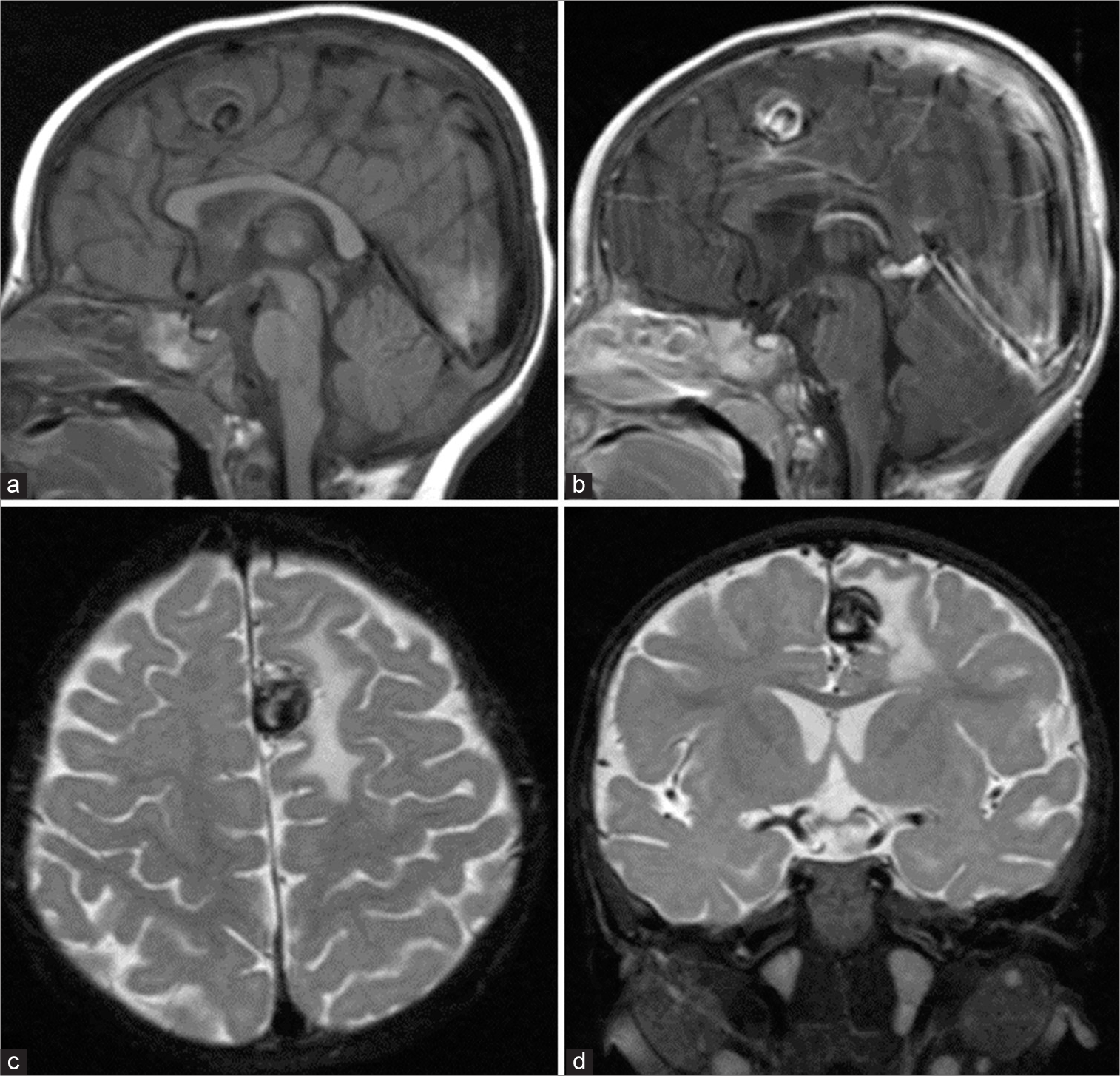
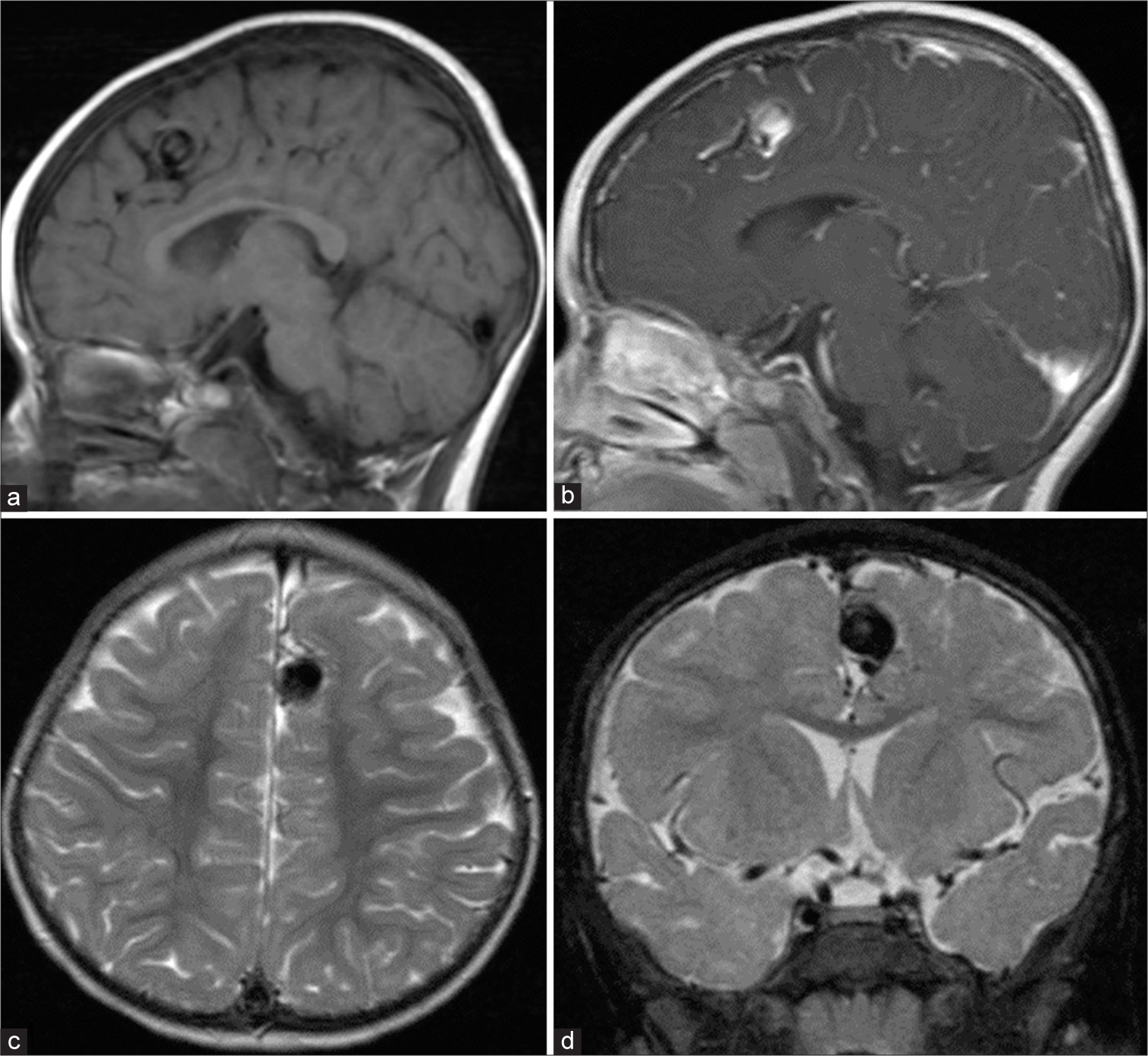
Six years after symptom onset, at age 7, a follow-up MRI and contrasted MRA confirmed the complete obliteration of the pial AVM and the intranidal aneurysm [
Figure 6:
At age 7. (a) Axial T2-weighted magnetic resonance imaging (MRI) reveals a decrease in the size of the aneurysm (arrow) at the medial part of the left superior frontal gyrus. (b) sagittal T1-weighted contrast-enhanced MRI demonstrates complete thrombosis of the large aneurysm (arrowhead). (c) Sagittal maximum intensity projection image from 3D time-of-flight magnetic resonance angiography confirms complete obliteration of the pial arteriovenous malformation and large intranidal aneurysm.
At age 10, follow-up cerebral angiography disclosed a constellation of small, loosely packed vessels in the vicinity of the previously identified large thrombosed intranidal aneurysm. This area, devoid of early draining veins, was suggestive of focal hyperemia [
Figure 7:
At age 10. (a) Axial, (b) coronal, and (c) sagittal views of maximum intensity projection reformatted image of angiographic computerized tomography, and (d) anteroposterior and (e) lateral views of the left internal carotid artery demonstrate a group of loosely packed tiny vessels (arrowheads) surrounding previous area of large thrombosed intranidal aneurysm without early draining vein, probably focal hyperemia. Arrowheads represent a group of loosely packed tiny vessels in all Figures 7a-7e.
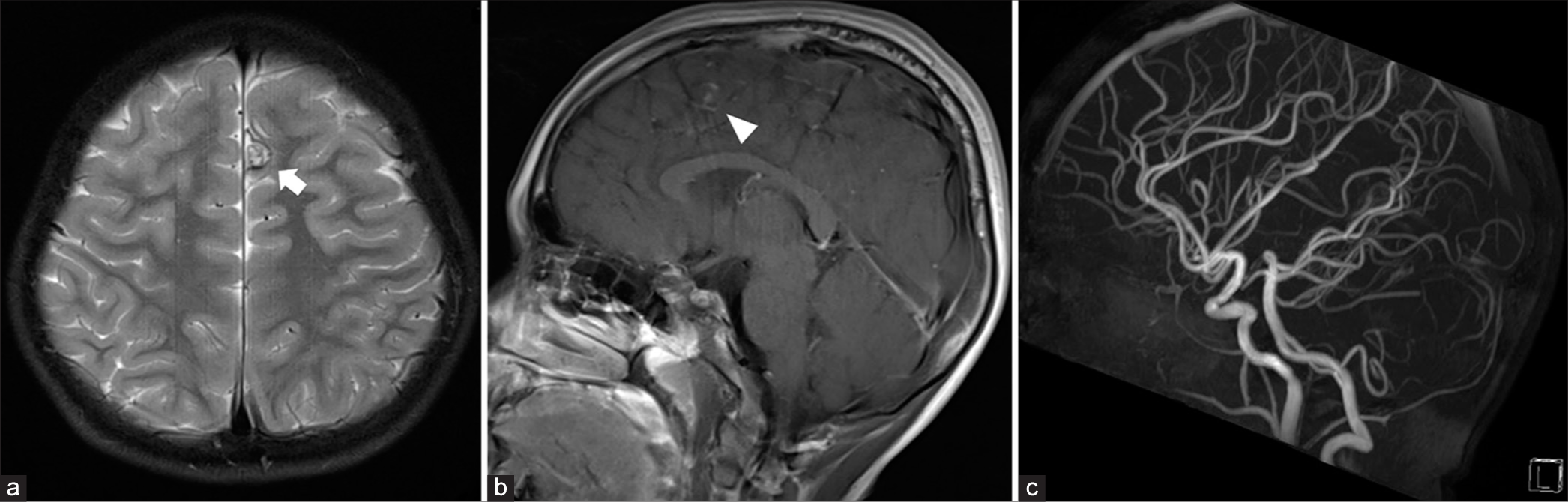
Figure 8:
At age 12. (a) Axial T2-weighted magnetic resonance imaging (MRI) shows a further reduction in the size of the aneurysm (arrow) at the medial part of the left superior frontal gyrus. (b) Coronal view of contrast-enhanced magnetic resonance angiographic source image, and (c) axial and (d) coronal views of T1-weighted contrast-enhanced MRI reveal enhancing area (arrowheads) surrounding previous area of large thrombosed intranidal aneurysm, probably focal hyperemia, corresponding with prior cerebral angiography.
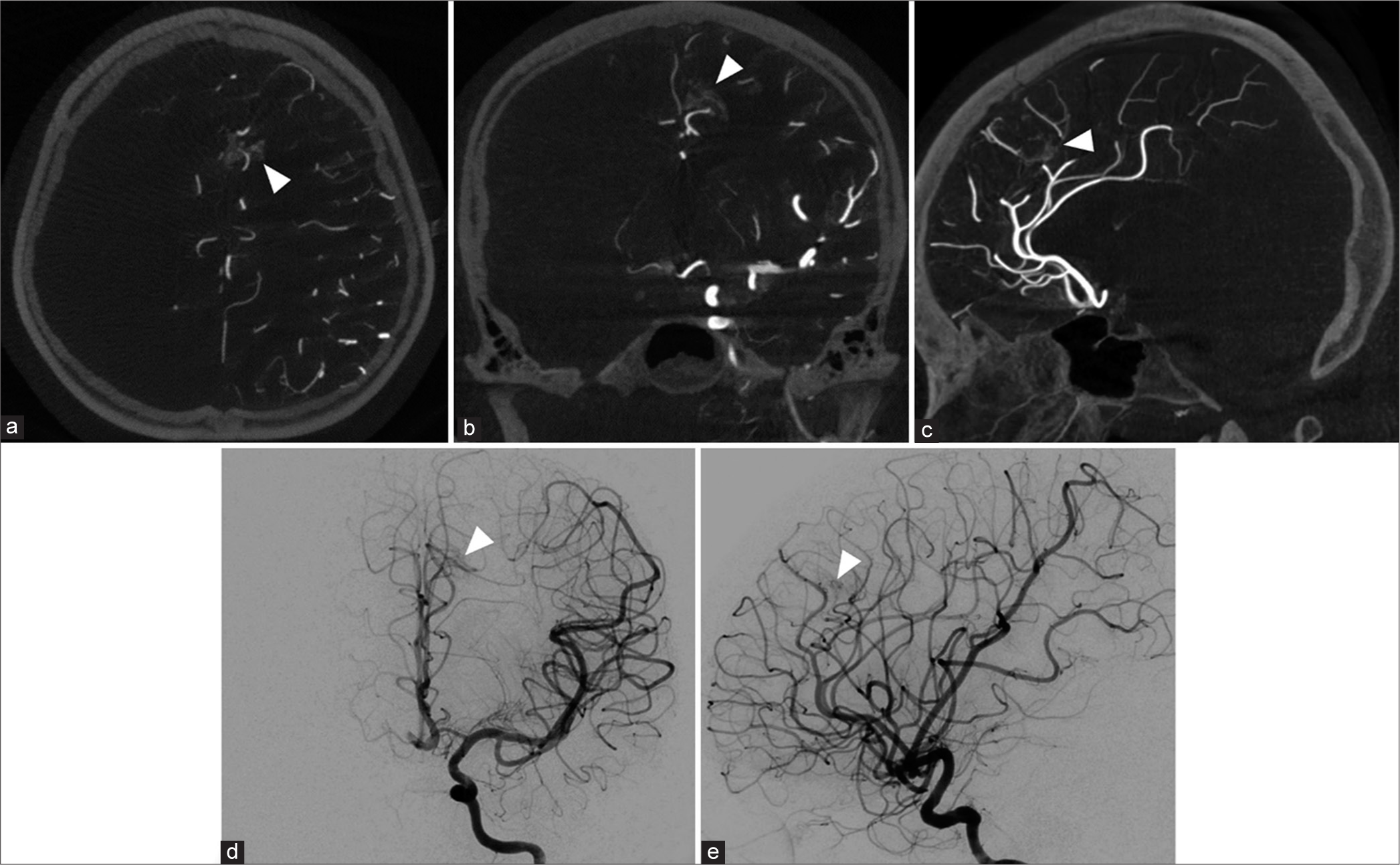
By age 15, the patient had completed her middle school education but decided not to pursue further studies in high school. Around this time, she was diagnosed with depressive mood disorder and commenced treatment with sertraline at a dose of 50 mg/day. At age 16, further imaging was undertaken, including a 3 Tesla cranial MRI and 4D dynamic contrast-enhanced MRA, which identified a recurrent pial AVM located in the left superior frontal region [
Figure 9:
Imaging findings at 16 years of age. (a) Three-dimensional raw time-of-flight magnetic resonance angiography (MRA) source image demonstrates a cluster of diminutive vessels featuring an early draining vein (indicated by an arrow) located in the left superior frontal region. (b) Anteroposterior (AP) projection and (c) lateral projection of maximum-intensity 4D contrast-enhanced MRA reveal a recurrent pial arteriovenous malformation (AVM) characterized by an early draining vein (marked by white arrowheads). (d) AP and (e) lateral views of the left internal carotid artery injection confirm the presence of a recurrent pial AVM with an early draining vein (denoted by black arrowheads).
Subsequent cerebral angiography performed 1 week later corroborated the findings of a recurrent pial AVM [
DISCUSSION
Pediatric cerebral AVMs, primarily manifesting as hemorrhage in over 60% of cases, predominantly affect adolescents rather than younger children, with a rarity in infancy.[
The primary treatment goal for pediatric ruptured brain AVMs is complete obliteration, preserving neurological function, and preventing further bleeding incidents.[
AVMs with associated aneurysms, found in 29% of pediatric cases, present a higher risk of rebleeding. The management of AVM-associated aneurysms, especially arterial ones, should not be delayed due to their strong correlation with hemorrhage.[
Spontaneous obliteration of untreated cerebral AVMs is a rare phenomenon, and the incidence of this phenomenon was <1%.[
Spontaneous regression of pediatric AVMs is exceedingly rare, particularly in children under 5 years.[
To the best of our knowledge, there was only one previous report of spontaneous disappearance of infant cerebral AVM by Mabe and Furuse.[
At present, two cases of spontaneous resolution of cerebral pial AVFs in children have been reported. The first case, described by Sabrina et al.,[
Interestingly, persistent focal hyperemia was observed during follow-up in our case, involving a patient aged 10–12, through control angiography and serial MRI/MRA examinations. The underlying pathophysiology of focal hyperemia, however, remains not fully understood. Neuronal activity in the brain triggers localized increases in blood flow, a phenomenon known as functional hyperemia. Essentially, when there is a surge in brain activity in a particular area, blood flow to that specific region also increases. This ensures that active neurons receive the necessary nutrients. Functional hyperemia refers to this elevation in local blood flow, leading to the dilation of blood vessels, as a direct response to neuronal activity.[
Focal hyperemia often follows traumatic brain injury, particularly in patients with focal contusions and intracerebral hematomas, affecting both gray and white matter. This hyperemia typically arises in the healthy tissue directly adjacent to focal mass lesions. During the healing process, neovascularization occurs around the contused brain area. However, these new blood vessels lack tight endothelial junctions, leading to disturbances in the blood-brain barrier. This type of focal and persistent hyperemia is considered a benign form of hyperemia. It generally has a minimal impact on intracranial pressure and the patient’s level of consciousness and is associated with a better patient outcome.[
Recurrent cerebral AVMs after angiographically documented complete excision may develop particularly in children, highlighting the importance of long-term imaging follow-up. Even though the likelihood of recurrence following a negative postoperative angiogram is low, the associated morbidity and mortality due to hemorrhage are significant.[
In the present case, the transition from focal hyperemia to the recurrence of a pial AVM was observed, illustrating a rare and clinically significant progression. Initially, focal hyperemia was noted in the regions surrounding the previously obliterated AVM. Typically, focal hyperemia indicates increased blood flow that can occur in response to localized neuronal activation or as a reactive process following vascular injury. In this case, it was hypothesized to be an adaptive response to the altered hemodynamics postinitial AVM obliteration. Over time, this area of hyperemia began to show signs of neovascularization. Neovascularization following AVM treatment, particularly in cases involving radiosurgery or surgical interventions, is well-documented, but its progression to AVM recurrence is not well understood and rarely documented. The development of a recurrent AVM from focal hyperemia in our patient underscores a critical aspect of pediatric AVM management: it particularly highlights the potential for angiogenic stimuli within the brain to promote the formation of abnormal vascular networks. Advanced imaging techniques, including dynamic contrast-enhanced MRA, were instrumental in identifying and monitoring these changes. These imaging findings were pivotal in distinguishing between benign hyperemia, which typically resolves without clinical sequelae, and the more concerning pattern suggestive of neovascular activity leading to AVM recurrence.
CONCLUSION
We reported an exceptionally rare case of spontaneous regression in a pial AVM concurrent with a large intranidal aneurysm. Extensive long-term follow-up and sequential imaging have meticulously documented the evolution and eventual complete thrombosis of both the substantial partially thrombosed aneurysm and the smaller pial AVM. This spontaneous regression was notably influenced by factors such as a hemorrhagic presentation, superficial venous drainage, and a small nidus. In addition, our observations confirmed the complete resolution of perianeurysmal edema.
It is crucial to recognize that spontaneous thrombosis of ruptured cerebral AVMs remains a rare and unpredictable phenomenon. Therefore, it should not be considered a reliable treatment strategy in the management of brain AVMs. Instead, meticulous long-term clinical and radiological monitoring is imperative for pediatric patients who experience spontaneous obliteration of cerebral AVMs.
Moreover, our case provides a significant example of focal hyperemia developing into a recurrent pial AVM, illustrating a dynamic and critical area of pediatric neurovascular care. This progression prompts the need for further research and could potentially impact future treatment approaches and monitoring protocols in pediatric neurovascular disorders.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abdulrauf SI, Malik GM, Awad IA. Spontaneous angiographic obliteration of cerebral arteriovenous malformations. Neurosurgery. 1999. 44: 280-7
2. Andaluz N, Myseros JS, Sathi S, Crone KR, Tew JM. Recurrence of cerebral arteriovenous malformations in children: Report of two cases and review of the literature. Surg Neurol. 2004. 62: 324-30 discussion 330-1
3. Anderson RC, McDowell MM, Kellner CP, Appelboom G, Bruce SS, Kotchetkov IS. Arteriovenous malformation-associated aneurysms in the pediatric population. J Neurosurg Pediatr. 2012. 9: 11-6
4. Blauwblomme T, Bourgeois M, Meyer P, Puget S, Di Rocco F, Boddaert N. Long-term outcome of 106 consecutive pediatric ruptured brain arteriovenous malformations after combined treatment. Stroke. 2014. 45: 1664-71
5. Buis DR, van den Berg R, Lycklama G, van der Worp HB, Dirven CM, Vandertop WP. Spontaneous regression of brain arteriovenous malformations--a clinical study and a systematic review of the literature. J Neurol. 2004. 251: 1375-82
6. Chen KS, Williams DD, Iacobas I, McClugage SG, Gadgil N, Kan P. Spontaneous thrombosis of high flow pediatric arteriovenous fistulae: Case series of two patients and a comprehensive literature review. Childs Nerv Syst. 2024. 40: 1405-14
7. Dyck P. Spontaneous thrombosis of an arteriovenous malformation. Neurosurgery. 1977. 1: 287-90
8. El-Ghanem M, Kass-Hout T, Kass-Hout O, Alderazi YJ, Amuluru K, Al-Mufti F. Arteriovenous malformations in the pediatric population: Review of the existing literature. Interv Neurol. 2016. 5: 218-25
9. Fullerton HJ, Achrol AS, Johnston SC, McCulloch CE, Higashida RT, Lawton MT. Long-term hemorrhage risk in children versus adults with brain arteriovenous malformations. Stroke. 2005. 36: 2099-104
10. Fumeya H, Fujisaki I. Focal cerebral hyperemia. J Neurosurg. 1996. 84: 1079-80
11. Goyal N, Hoit D, Elijovich L. Spontaneous thrombosis of a ruptured brain arteriovenous malformation: The argument for early conservative management. Interv Neurol. 2015. 3: 122-8
12. Iampreechakul P, Tirakotai W, Tanpun A, Wattanasen Y, Lertbusayanukul P, Siriwimonmas S. Spontaneous resolution of direct carotid-cavernous fistulas: Case series and literature review. Interv Neuroradiol. 2019. 25: 71-89
13. Iampreechakul P, Wangtanaphat K, Hangsapruek S, Wattanasen Y, Lertbutsayanukul P, Siriwimonmas S. Acquired Chiari malformation type I and holocord syringomyelia associated with a high-flow supratentorial fistulous arteriovenous malformations: A case report and literature review. Surg Neurol Int. 2022. 13: 217
14. Iampreechakul P, Wangtanaphat K, Tirakotai W, Lertbutsayanukul P, Wattanasen Y, Hangsapruek S. Spontaneous thrombosis of a ruptured pial arteriovenous malformation and an associated large intranidal aneurysm with perianeurysmal edema in an infant: A case report. OMICS J Radiol. 2019. 8: 309
15. Iizuka Y, Rodesch G, Garcia-Monaco R, Alvarez H, Burrows P, Hui F. Multiple cerebral arteriovenous shunts in children: Report of 13 cases. Childs Nerv Syst. 1992. 8: 437-44
16. Jimenez JE, Gersey ZC, Wagner J, Snelling B, Ambekar S, Peterson EC. Role of follow-up imaging after resection of brain arteriovenous malformations in pediatric patients: A systematic review of the literature. J Neurosurg Pediatr. 2017. 19: 149-56
17. Kader A, Goodrich JT, Sonstein WJ, Stein BM, Carmel PW, Michelsen WJ. Recurrent cerebral arteriovenous malformations after negative postoperative angiograms. J Neurosurg. 1996. 85: 14-8
18. Krings T, Geibprasert S, Terbrugge K. Classification and endovascular management of pediatric cerebral vascular malformations. Neurosurg Clin N Am. 2010. 21: 463-82
19. Lasjaunias P, Hui F, Zerah M, Garcia-Monaco R, Malherbe V, Rodesch G. Cerebral arteriovenous malformations in children. Management of 179 consecutive cases and review of the literature. Childs Nerv Syst. 1995. 11: 66-79
20. Lee SK, Vilela P, Willinsky R, TerBrugge KG. Spontaneous regression of cerebral arteriovenous malformations: Clinical and angiographic analysis with review of the literature. Neuroradiology. 2002. 44: 11-6
21. Leung KM, Agid R, terBrugge K. Spontaneous regression of a cerebral arteriovenous malformation in a child with hereditary hemorrhagic telangiectasia. Case report. J Neurosurg. 2006. 105: 428-31
22. Liew JA, Yang W, Mashouf LA, Li S, Caplan JM, Tamargo RJ. Incidence of spontaneous obliteration in untreated brain arteriovenous malformations. Neurosurgery. 2020. 86: 139-49
23. Mabe H, Furuse M. Spontaneous disappearance of a cerebral arteriovenous malformation in infancy. Case report. J Neurosurg. 1977. 46: 811-5
24. Mizutani T, Tanaka H, Aruga T. Total recanalization of a spontaneously thrombosed arteriovenous malformation. Case report. J Neurosurg. 1995. 82: 506-8
25. Morgan MK, Patel NJ, Simons M, Ritson EA, Heller GZ. Influence of the combination of patient age and deep venous drainage on brain arteriovenous malformation recurrence after surgery. J Neurosurg. 2012. 117: 934-41
26. Nippert AR, Biesecker KR, Newman EA. Mechanisms mediating functional hyperemia in the brain. Neuroscientist. 2018. 24: 73-83
27. Nukui H, Miyagi O, Tamada J, Mitsuka S, Kawafuchi J. Long-term follow-up study by cerebral angiography in cases with arteriovenous malformation of the brain. With special reference to spontaneous disappearance of arteriovenous malformation in cerebral angiography. Neurol Med Chir (Tokyo). 1982. 22: 125-32 [Article in Japanese]
28. Patel MC, Hodgson TJ, Kemeny AA, Forster DM. Spontaneous obliteration of pial arteriovenous malformations: A review of 27 cases. AJNR Am J Neuroradiol. 2001. 22: 531-6
29. Sabrina C, Laura M, Sabrina S, Giacomo T, Nicoletta M, Elena P. Expect the unexpected: A case of spontaneous thrombosis of a pial arteriovenous fistula in a preterm newborn with review of the literature. Childs Nerv Syst. 2023. 39: 793-9
30. Sakas DE, Bullock MR, Patterson J, Hadley D, Wyper DJ, Teasdale GM. Focal cerebral hyperemia after focal head injury in humans: A benign phenomenon?. J Neurosurg. 1995. 83: 277-84
31. Shtaya A, Millar J, Sparrow O. Multimodality management and outcomes of brain arterio-venous malformations (AVMs) in children: Personal experience and review of the literature, with specific emphasis on age at first AVM bleed. Childs Nerv Syst. 2017. 33: 573-81
32. Yu S, Yan L, Yao Y, Wang S, Yang M, Wang B. Noncontrast dynamic MRA in intracranial arteriovenous malformation (AVM), comparison with time of flight (TOF) and digital subtraction angiography (DSA). Magn Reson Imaging. 2012. 30: 869-77