- Department of Neurosurgery, Keio University, School of Medicine,
- Biostatistics Unit, Clinical and Translational Research Center, Keio University Hospital, Tokyo, Japan.
Correspondence Address:
Satoshi Takahashi
Department of Neurosurgery, Keio University, School of Medicine,
DOI:10.25259/SNI_551_2019
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Satoshi Takahashi, Takenori Akiyama, Takashi Horiguchi, Tomoru Miwa, Ryo Takemura, Kazunari Yoshida. Loss of consciousness at ictus and/or poor World Federation of Neurosurgical Societies grade on admission reflects the impact of EBI and predicts poor outcome in patients with SAH. 06-Mar-2020;11:40
How to cite this URL: Satoshi Takahashi, Takenori Akiyama, Takashi Horiguchi, Tomoru Miwa, Ryo Takemura, Kazunari Yoshida. Loss of consciousness at ictus and/or poor World Federation of Neurosurgical Societies grade on admission reflects the impact of EBI and predicts poor outcome in patients with SAH. 06-Mar-2020;11:40. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=9890
Abstract
Background: There are many scores and markers that predict poor outcome in patients with subarachnoid hemorrhage (SAH). However, parameters that can predict outcomes in patients with SAH with high specificity and sensitivity, which can be identified in the early postictal state and utilized as a clinical marker of early brain injury (EBI) have not been identified so far.
Methods: Thirty-nine patients with SAH due to a saccular intracranial aneurysm rupture were reviewed. We retrospectively analyzed the relationships between patients’ baseline characteristics and patients’ outcomes to identify parameters that could predict patient outcomes in the early postictal state.
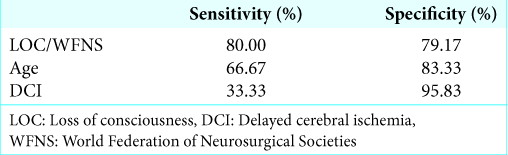
Results: In the univariate analysis, older age (>65), loss of consciousness (LOC) at ictus, poor initial World Federation of Neurosurgical Societies (WFNS) grade (3–5), and delayed cerebral ischemia (DCI) were associated with poor outcome (GOS 1–3). Statistical analyses revealed that combined LOC at ictus and/or poor initial WFNS grade (3–5) was a more powerful surrogate marker of outcome (OR 15.2 [95% CI 3.1–75.5]) than either LOC at ictus or the poor initial WFNS grade (3–5) alone. Multivariate logistic regression analyses revealed that older age, combined LOC at ictus and/or poor initial WFNS grade, and DCI were independently associated with poor outcome.
Conclusion: Combined LOC at ictus and/or poor initial WFNS grade (3–5) reflects the impact of EBI and was a useful surrogate marker of poor prognosis in SAH patients, independent of patients’ age and state of DCI.
Keywords: Early brain injury, Loss of consciousness, Outcome, Subarachnoid hemorrhage, World Federation of Neurosurgical Societies grade
INTRODUCTION
Despite the development of modern neurosurgery, the morbidity and mortality of patients who have suffered from subarachnoid hemorrhage (SAH) due to a ruptured intracranial aneurysm remain high. Survival from aneurysmal SAH has increased by 17% in the past few decades; however, SAH still leads to the loss of many years of productive life.[
If the surgical obliteration of a ruptured aneurysm by means of clipping or coiling is performed successfully, delayed cerebral ischemia (DCI) often occurs 3–14 days after SAH onset (during the so-called “vasospasm period”), and the neurological functions of patients deteriorate.[
Recently, the concept of early brain injury (EBI) has emerged, and its relationship to DCI has attracted attention. EBI is the term used to describe the pathophysiological events between days 0 and 3 or 4 that induce immediate injury to the brain.[
The above-mentioned damage resulting from EBI due to SAH seems to be difficult to directly evaluate in patients by means of objective parameters. In the present study, we retrospectively analyzed the relationships between patients’ baseline characteristics that are identifiable in the early postictal state and patients’ outcomes to identify parameters that can predict patient outcome and reflect the impact of EBI.
MATERIALS AND METHODS
Patients populations
We retrospectively analyzed data from 39 adult patients with documented SAH due to the rupture of a saccular intracranial aneurysm, who were treated at our institution in the 3.5-year period since January 2015. Patients with SAH due to cerebral artery dissection or poor-grade SAH, who could not be surgically treated were excluded from the study. Complete medical and neurological examinations, as well as neuroimaging scans including magnetic resonance angiography or 3D computed tomography (CT) angiography, were performed on all patients. All patients included in the current study underwent clipping/coiling (stratified by site according to aneurysmal location and shape) within 72 h of onset. This study was approved by the Institutional Review Board of Keio University, School of Medicine, with the accession number 20140327. This study was performed in compliance with the Declaration of Helsinki.
Candidate parameters
Patients’ characteristics such as age, gender, clinical LOC status at ictus, Subarachnoid Hemorrhage Early Brain Edema Score (SEBES), size of aneurysm, surgical strategy (clip or coil), World Federation of Neurosurgical Societies (WFNS) grade on admission, cerebral vasospasm, and DCI were initially selected. Of these parameters, LOC at ictus, SEBES, and WFNS grade on admission were selected as possible parameters that could reflect EBI. For data assessment, a SEBES of 3 or 4 was regarded as a high SEBES according to the previous reports.[
WFNS grade of 3–5 was regarded as a poor grade. Radiological vasospasm and DCI, which are known as poor prognostic factors in patients with SAH, were also assessed for multiple regression analysis. A GOS of 1–3 at the time of discharge was considered to be a poor outcome in the present study.
Definition of vasospasm and DCI in the present study
In the present study, we found that vasospasm was present if moderate (34–66%) or severe (67–100%) vasospasm, as described previously, was present within 4–14 days from ictus.[
Statistical analysis
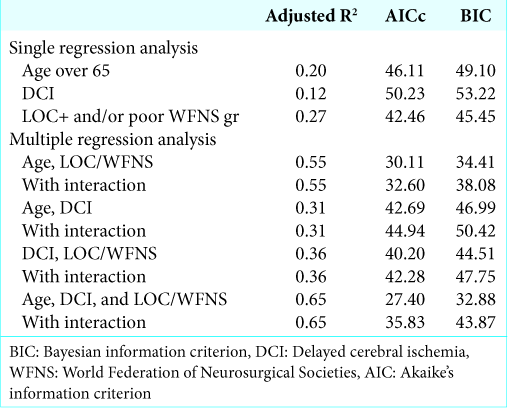
We statistically analyzed the relationships between the baseline characteristics of the patients and patient outcomes. Baseline characteristics were compared between the different GOS groups using the Chi-square test for nominal variables and logistic regression analysis for continuous variables (i.e., age and the diameter of the aneurysm). Multivariate logistic regression analyses were also performed to account for patient characteristics and clinical parameters that were statistically significant in univariate analysis. Model selection using an adjusted R2, Akaike’s information criterion with a correction for small sample sizes Akaike’s information criterion (AICc), and the Bayesian information criterion (BIC) was also performed. P = 0.05 was considered statistically significant. For continuous variables, when statistically significant differences were identified between parameters, the cutoff points were calculated with receiver operating characteristic (ROC) curves.
RESULTS
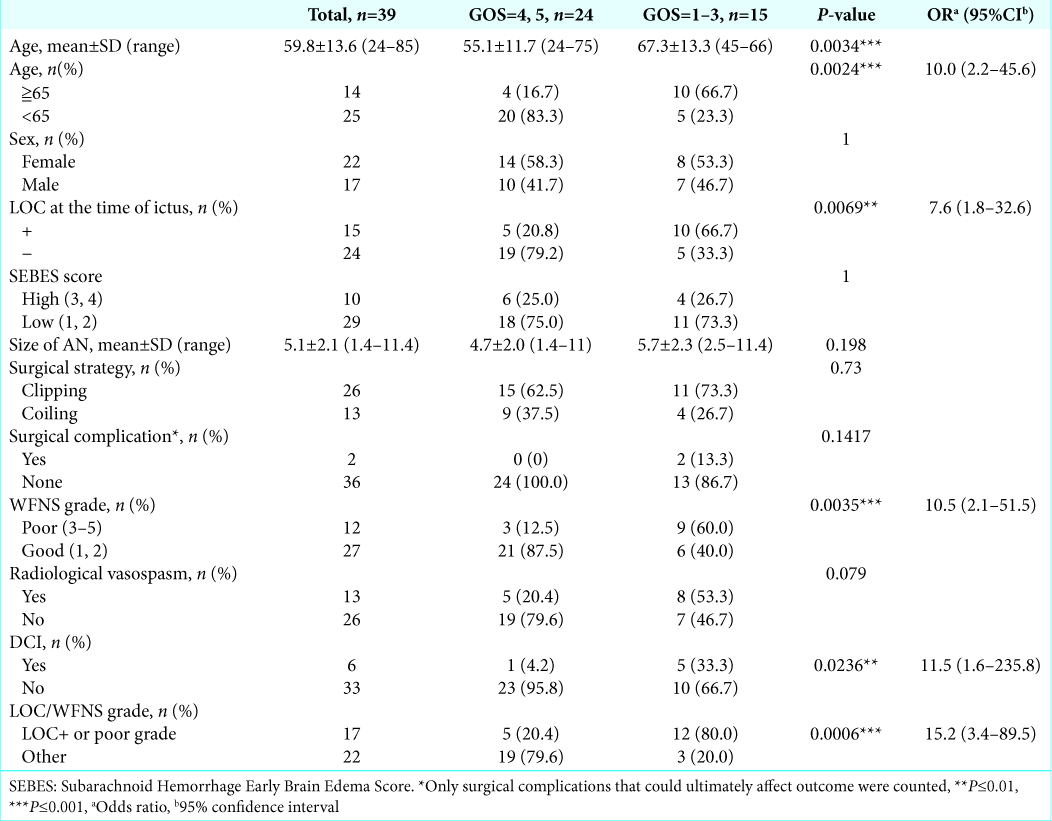
Patient characteristics
The patient group consisted of 22 females and 17 males, and the mean age was 59.8 years (range, 24–85 years). The treatment strategies were mainly determined at the site by three on-call attending neurosurgeons (ST, TA, and TM) according to the location and shape of the ruptured aneurysm, since in Japan, the same vascular neurosurgeon does both clipping and coiling. Twenty-six aneurysms in 26 patients were clipped, and the other 13 aneurysms in 13 patients were coiled. The baseline characteristics of the patients are summarized in
Parameters that predict patient outcome
Age
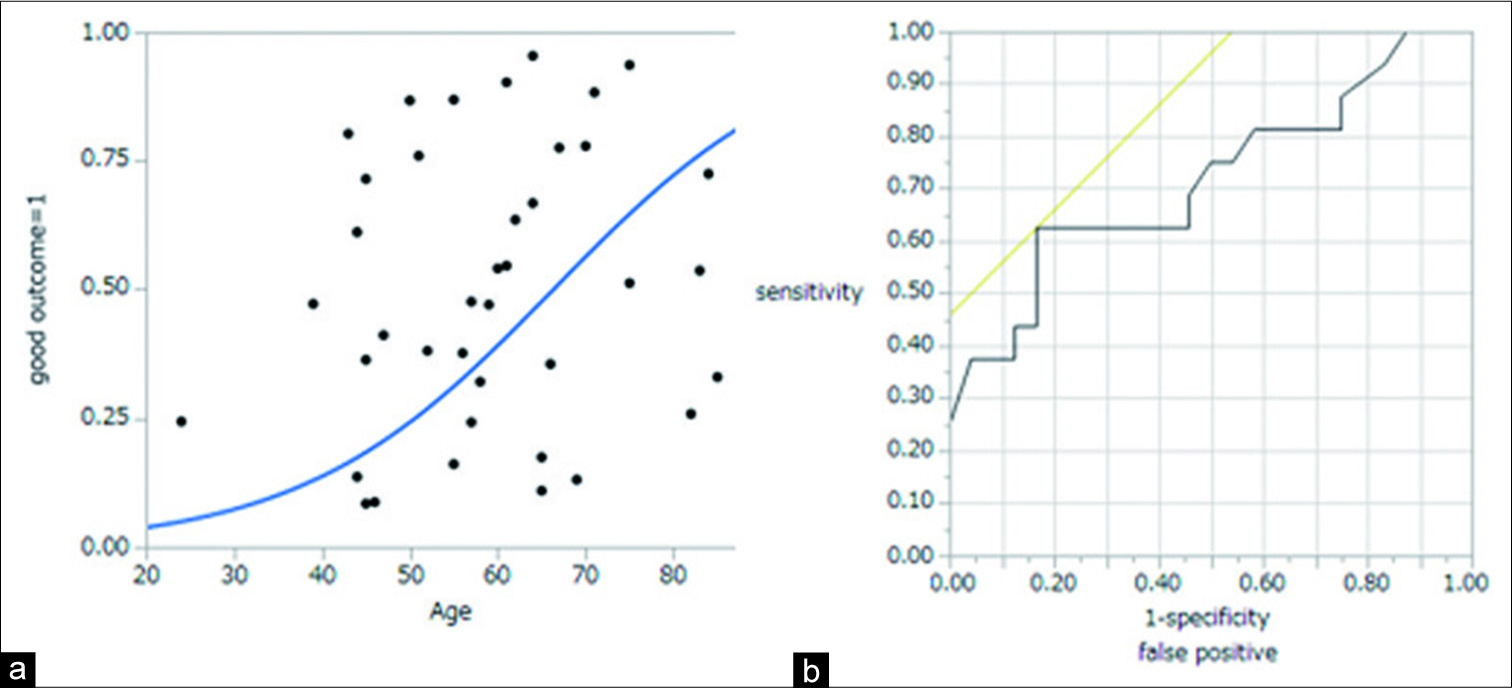
A logistic regression analysis showed that age was significantly associated with outcome. This result showed that the older patient was, the worse the patient outcome would be. Then, ROC curve analysis was used to determine the optimal cutoff point for age predicting poor outcome. The ROC curve for predicting poor outcome revealed that the area under the curve (AUC) value for age was 0.753, and the analysis identified an optimal cutoff point as 65 years old, with 66.7% sensitivity and 50.0% specificity [
Figure 1:
(a) Logistic regression analysis showed that age was significantly associated with outcome. The result showed that the older patient was, the worse the outcome would be. (b) The receiver operating characteristic curve for predicting poor outcome revealed that the area under the curve value for age was 0.753, and the analysis identified an optimal cutoff point of 65 years old.
Diameter of aneurysm
In the present study, the diameter of the aneurysm was not significantly associated with the outcome according to logistic regression analysis.
Nominal variables
In the univariate analysis, older age (≥65), LOC at ictus, poor WFNS grade (3–5), and DCI were statistically significant prognostic factors in patients with SAH. Surgical complications were occurred in two out of 39 patients analyzed; however, it did not affect outcome statistically. The odds ratio (OR) and P values of each variable are summarized in
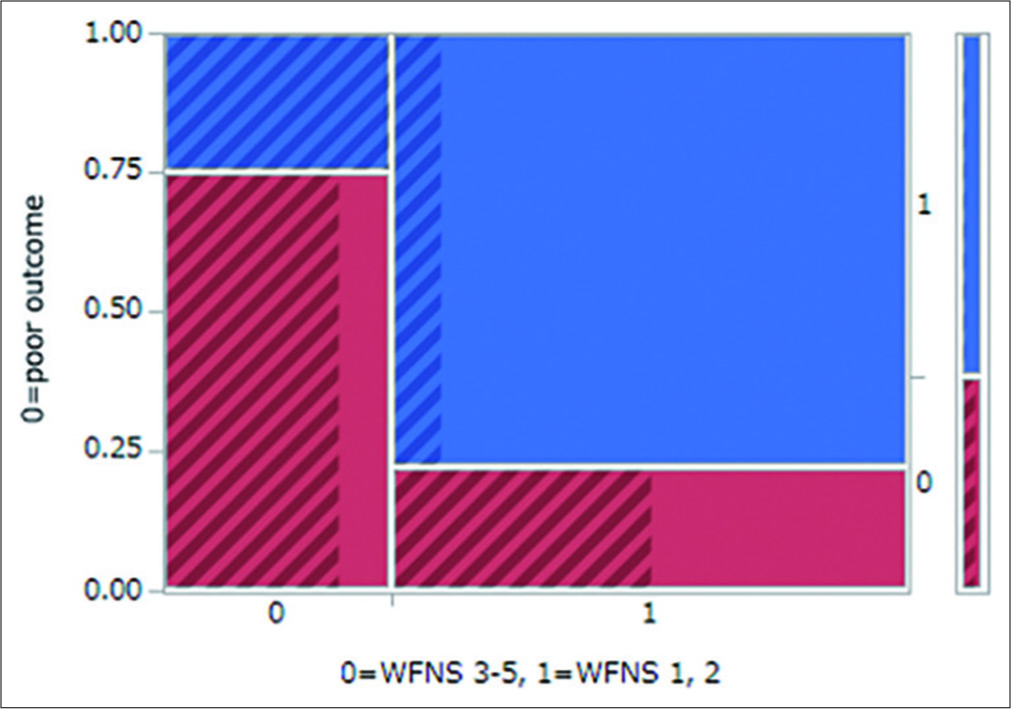
Statistically significant variables that can be identified in the early stage after ictus
LOC at ictus and a poor initial WFNS grade were both statistically significant variables that could be identified in the early postictal state. These are factors that reflect initial brain damage due to ictus. Thus, to avoid confounding factors, we constructed a mosaic plot showing the relationships between WFNS grade and outcome, with overlaying LOC status (
Multivariate logistic regression analysis
Multivariate logistic regression analysis was initially performed using three parameters: age, combined LOC at ictus and/or poor WFNS grade, and DCI. However, due to the small sample size, the analysis did not converge. Therefore, we adopted the model selection method and calculated the adjusted R2, AICc, and BIC of every two or three pairs of parameters, with or without accounting for interaction [
DISCUSSION
In the present study, we found that combined LOC at ictus and/or poor initial WFNS grade is a useful prognostic marker that can be identified in the early postictal state with 80.00% sensitivity and 79.17% specificity. Multiple regression analysis with model selection methods revealed that combined LOC at ictus and/or poor initial WFNS grade is a prognostic marker that is independent of patient age and the state of DCI. This fact showed that the prognoses of SAH patients have already been determined, to some extent, as early as the time of admission. We will thus interpret the result that combined LOC at ictus and/or poor initial WFNS grade reflects the impact of EBI.
EBI causes subsequent damage to the brain. Global brain ischemia due to intracranial hypertension following aneurysmal rupture causes the activation of miscellaneous pathways, including inflammatory, oxidative stress, apoptotic, and cortical spreading depolarization pathways, among others.[
The previous studies have already reported LOC at ictus as a predictor of clinical outcome. Suwatcharangkoon et al. analyzed the data of 1460 consecutively treated patients with SAH and reported that 40.4% of them experienced LOC at the onset of SAH.[
SAHIT is a tool that was developed for the reliable estimation of SAH outcomes.[
Many other useful outcome predictors have also been confirmed in patients with SAH. Faust et al. reported that a mean arterial blood pressure elevated >25% within the 1st week after SAH was associated with an unfavorable outcome.[
To screen, the patients who are considerably impacted by EBI are important so we can provide more prudent care to these patients. However, even if we could screen for these kinds of patients, improvement in the clinical outcome of these patients could not be achieved without first elucidating the mechanism of EBI and developing a new medical approach for EBI. To achieve this goal, our group is now working on basic research to elucidate the molecular mechanism of EBI. In particular, we are now trying to identify microRNAs that are specifically overexpressed or downregulated by EBI that can be separated from exosomes within the patients’ peripheral blood samples to serve as a clinical marker of EBI (data not shown). After the initial rupture of the aneurysm, abnormalities such as dysfunction in autoregulation, clot degeneration products, neuroinflammation, and hyponatremia/endocrine dysfunction occur.[
The current study also has certain limitations. The number of patients included in the current study was relatively small. The small study sample prevented us from conducting multivariate analysis with three parameters at 1 time; however, model selection suggested that combined LOC at ictus and/or poor WFNS grade was a useful predictor of the prognoses of patients with SAH, independent of age or DCI.
CONCLUSION
Combined LOC at ictus and/or poor initial WFNS grade is a useful surrogate marker of poor prognosis in SAH patients that is independent of patient age and the state of DCI. The results revealed that the prognoses of patients with SAH are largely affected by brain damage in the early postictal state (i.e., EBI). The influence of EBI on outcome in patients with SAH has emerged along with the development of modern treatment strategies that prevent vasospasm. Understanding the pathology of EBI, as well as developing new therapeutic and diagnostic strategies to prevent EBI, will be important in future.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
References
1. Ahn SH, Savarraj JP, Pervez M, Jones W, Park J, Jeon SB. The subarachnoid hemorrhage early brain edema score predicts delayed cerebral ischemia and clinical outcomes. Neurosurgery. 2018. 83: 137-45
2. Egashira Y, Zhao H, Hua Y, Keep RF, Xi G. White matter injury after subarachnoid hemorrhage: Role of blood-brain barrier disruption and matrix metalloproteinase-9. Stroke. 2015. 46: 2909-15
3. Fang R, Zheng X, Zhang M. Ethyl pyruvate alleviates early brain injury following subarachnoid hemorrhage in rats. Acta Neurochir (Wien). 2016. 158: 1069-76
4. Faust K, Horn P, Schneider UC, Vajkoczy P. Blood pressure changes after aneurysmal subarachnoid hemorrhage and their relationship to cerebral vasospasm and clinical outcome. Clin Neurol Neurosurg. 2014. 125: 36-40
5. Fragata I, Alves M, Papoila AL, Nunes AP, Ferreira P, Canto-Moreira N. Early prediction of delayed ischemia and functional outcome in acute subarachnoid hemorrhage: Role of diffusion tensor imaging. Stroke. 2017. 48: 2091-7
6. Fujii M, Yan J, Rolland WB, Soejima Y, Caner B, Zhang JH. Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl Stroke Res. 2013. 4: 432-46
7. Fujimoto M, Shiba M, Kawakita F, Liu L, Shimojo N, Imanaka-Yoshida K. Deficiency of tenascin-C and attenuation of blood-brain barrier disruption following experimental subarachnoid hemorrhage in mice. J Neurosurg. 2016. 124: 1693-702
8. Hayman EG, Wessell A, Gerzanich V, Sheth KN, Simard JM. Mechanisms of global cerebral edema formation in aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2017. 26: 301-10
9. Jaja BN, Saposnik G, Lingsma HF, Macdonald E, Thorpe KE, Mamdani M. Development and validation of outcome prediction models for aneurysmal subarachnoid haemorrhage: The SAHIT multinational cohort study. BMJ. 2018. 360: j5745-
10. Kusaka G, Ishikawa M, Nanda A, Granger DN, Zhang JH. Signaling pathways for early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2004. 24: 916-25
11. Lagares A, Jiménez-Roldán L, Gomez PA, Munarriz PM, Castaño-León AM, Cepeda S. Prognostic value of the amount of bleeding after aneurysmal subarachnoid hemorrhage: A quantitative volumetric study. Neurosurgery. 2015. 77: 898-907
12. Macdonald RL, Kassell NF, Mayer S, Ruefenacht D, Schmiedek P, Weidauer S. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): Randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke. 2008. 39: 3015-21
13. Macdonald RL, Schweizer TA. Spontaneous subarachnoid haemorrhage. Lancet. 2017. 389: 655-66
14. Peeyush Kumar T, McBride DW, Dash PK, Matsumura K, Rubi A, Blackburn SL. Endothelial cell dysfunction and injury in subarachnoid hemorrhage. Mol Neurobiol. 2019. 56: 1992-2006
15. Sano H, Satoh A, Murayama Y, Kato Y, Origasa H, Inamasu J. Modified world federation of neurosurgical societies subarachnoid hemorrhage grading system. World Neurosurg. 2015. 83: 801-7
16. Savarraj J, Parsha K, Hergenroeder G, Ahn S, Chang TR, Kim DH. Early brain injury associated with systemic inflammation after subarachnoid hemorrhage. Neurocrit Care. 2018. 28: 203-11
17. Sokół B, Wąsik N, Więckowska B, Mańko W, Juszkat R, Jankowski R. Predicting mortality in subarachnoid haemorrhage based on first-week routine blood tests. J Clin Neurosci. 2018. 58: 100-7
18. Suwatcharangkoon S, Meyers E, Falo C, Schmidt JM, Agarwal S, Claassen J. Loss of Consciousness at onset of subarachnoid hemorrhage as an important marker of early brain injury. JAMA Neurol. 2016. 73: 28-35
19. Suzuki H, Kawakita F, Liu L, Nakano F. Delayed brain injury after aneurysmal subarachnoid hemorrhage: Update and perspective. No Shinkei Geka. 2015. 43: 869-77
20. Vartanian KB, Mitchell HD, Stevens SL, Conrad VK, McDermott JE, Stenzel-Poore MP. CpG preconditioning regulates miRNA expression that modulates genomic reprogramming associated with neuroprotection against ischemic injury. J Cereb Blood Flow Metab. 2015. 35: 257-66
21. Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: Proposal of a multidisciplinary research group. Stroke. 2010. 41: 2391-5
22. Wang J, Alotaibi NM, Akbar MA, Ayling OG, Ibrahim GM, Macdonald RL. Loss of Consciousness at onset of aneurysmal subarachnoid hemorrhage is associated with functional outcomes in good-grade patients. World Neurosurg. 2017. 98: 308-13
23. Ying GY, Jing CH, Li JR, Wu C, Yan F, Chen JY. Neuroprotective effects of valproic acid on blood-brain barrier disruption and apoptosis-related early brain injury in rats subjected to subarachnoid hemorrhage are modulated by heat shock protein 70/matrix metalloproteinases and heat shock protein 70/Akt pathways. Neurosurgery. 2016. 79: 286-95
24. Zoerle T, Lombardo A, Colombo A, Longhi L, Zanier ER, Rampini P. Intracranial pressure after subarachnoid hemorrhage. Crit Care Med. 2015. 43: 168-76