- Department of Neurosurgery, Dayton Children's Hospital, Wright State Boonshoft School of Medicine, Dayton, Ohio, United States
Correspondence Address:
R. Fujimura
Department of Neurosurgery, Dayton Children's Hospital, Wright State Boonshoft School of Medicine, Dayton, Ohio, United States
DOI:10.4103/sni.sni_444_17
Copyright: © 2018 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: R. Fujimura, R. Lober, K. Kamian, L. Kleiner. Maladjustment of programmable ventricular shunt valves by inadvertent exposure to a common hospital device. 01-Mar-2018;9:51
How to cite this URL: R. Fujimura, R. Lober, K. Kamian, L. Kleiner. Maladjustment of programmable ventricular shunt valves by inadvertent exposure to a common hospital device. 01-Mar-2018;9:51. Available from: http://surgicalneurologyint.com/surgicalint-articles/maladjustment-of-programmable-ventricular-shunt-valves-by-inadvertent-exposure-to-a-common-hospital-device/
Abstract
Background:Programmable ventricular shunt valves are commonly used to treat hydrocephalus. They can be adjusted to allow for varying amounts of cerebrospinal fluid (CSF) flow using an external magnetic programming device, and are susceptible to maladjustment from inadvertent exposure to magnetic fields.
Case Description:We describe the case of a 3-month-old girl treated for hydrocephalus with a programmable StrataTM II valve found at the incorrect setting on multiple occasions during her hospitalization despite frequent reprogramming and surveillance. We found that the Vocera badge, a common hands-free wireless communication system worn by our nursing staff, had a strong enough magnetic field to unintentionally change the shunt setting. The device is worn on the chest bringing it into close proximity to the shunt valve when care providers hold the baby, resulting in the maladjustment.
Conclusion:Some commonly used medical devices have a magnetic field strong enough to alter programmable shunt valve settings. Here, we report that the magnetic field from the Vocera hands-free wireless communication system, combined with the worn position, results in shunt maladjustment for the StrataTM II valve. Healthcare facilities using the Vocera badges need to put protocols in place and properly educate staff members to ensure the safety of patients with StrataTM II valves.
Keywords: Magnetic field, StrataTM programmable shunt, Vocera
INTRODUCTION
Programmable ventricular shunt valves allow for adjustable control of cerebrospinal fluid (CSF) flow using an external magnetic field. Orientation of valve mechanisms can generally be changed by a rotation that coordinates with the direction that the magnet is turned, modifying the opening pressure or rate of flow through the valve. This re-configuration mechanism allows for unrelated electronic devices with magnetic fields to change valve settings [
According to the manufacturer, Medtronic, a magnetic field must have a strength of at least 80 gauss (G) and be adjacent to the Strata™ II valve to move its mechanism.[
Although patient education is commonly provided about household items with magnetic fields, the risks of commonly used medical devices in the hospital can be overlooked.
CASE REPORT
The patient is a 3-month-old girl born premature at 33 weeks’ gestation with accelerated head growth and a bulging fontanelle from post-hemorrhagic hydrocephalus. Treatment with a right frontal ventriculoperitoneal shunt with a programmable Medtronic Strata™ II valve was selected because of extreme ventriculomegaly with a very thin cortical mantle and unpredictable CSF drainage needs. The patient underwent a postoperative magnetic resonance imaging (MRI), which showed decreased ventricular size and confirmed the appropriate function of the shunt. Following imaging, the shunt was programmed to performance level 2.5, and was confirmed to be at this setting seven days later. Three days later, the patient was fussy and feeding poorly. The valve was found to be set at performance level 1.0, and was reprogrammed to 2.5. The following day, it was again back at 1.0, and an investigation was started to determine the cause of the maladjustment.
Despite a lack of warnings regarding magnetic fields from the manufacturer, we hypothesized that the Vocera badge, a newly instituted device in our facility, might be responsible for the inadvertent valve changes. We found that sweeping a Vocera badge across a packaged Strata™ II valve caused the ball and valve mechanism to spin, changing the performance setting.
Upon further investigation, we learned that the patient's head was brought into an adjacent position to the Vocera badge by nursing staff when held for feedings. This proximity of the valve and badge caused changes to the valve's setting. A policy was instituted prohibiting Vocera badges in the patient's room. Alerts were also placed in the electronic medical system. After institution of the new policy, the patient's shunt setting was correct for the remainder of the hospitalization.
DISCUSSION
Programmable shunt valves allow control of CSF flow, which may be essential to a patient's treatment plan. In this particular case, it was important to regulate the outflow of the shunt to avoid over-drainage and possible collapse of the thin cortical mantle. For this patient and many others, awareness of external objects that may influence these settings is imperative. It is reported that 2% of programmable valve shunt complications are drainage related. As more electronic and technological advances make their way into everyday life, this number may increase.[
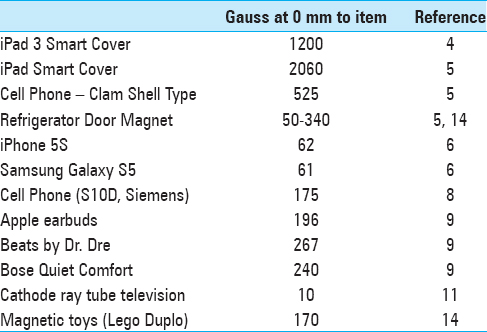
Medtronic Inc., the manufacturer of Strata™ valves, reports that 80 G directly adjacent to the valve is necessary for any adjustment to occur.[
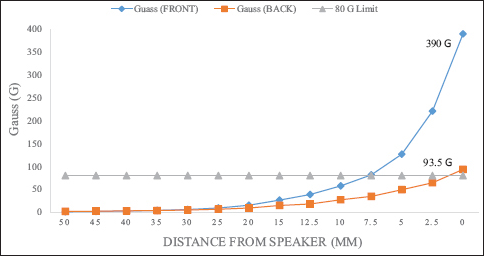
According to their internal testing, the Vocera badge speaker caused a change when dragged across the valve cassette mechanism where the magnetic rotor resides in the Strata™ valve. Medtronic found that the magnetic field of the Vocera badge was the same when powered on or off, and was the strongest in the center of the speaker. Both the front of the badge (450G) and the back (100G) are strong enough to provoke adjustment [
The manufacturer provides educational materials that warn of potential hazards due to the magnetic fields produced by common household items [
Other programmable valves hold similar risks. Manufacturers claim that Codman-Hakim valves require a minimum of 82 G to alter the setting.[
Vocera badges are used commonly in health care settings and produce magnetic fields sufficient to change the Medtronic Strata™ II valve. Vocera badges are hands-free, wearable, lightweight, voice-controlled devices that are utilized to communicate within a network.[
When discussing these potential risks with Vocera, they released a statement stating the magnetic field was indeed strong enough to influence the programmable valves. They also recommended that the badges not be brought within 2 inches of the valves. Suggestions were made to wear Vocera on the upper arm, close to the clavicle, or removing it from personnel before entering the room to prevent interference.
CONCLUSION
Many household items have been found to interfere with the programmable shunt valves. Anything that creates a strong enough magnetic field could potentially influence the setting. This includes devices within the healthcare environment that may not be recognized as safety concerns. As more technological devices are made and implemented in hospitals, it should be noted that a potential interference with care of magnetically controlled devices exists. Restriction of Vocera badges in rooms with patients with programmable ventricular valve shunts may be necessary. Further investigation may be needed to discover effects on other medical devices as well.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ahn ES, Bookland M, Carson BS, Weingart JD, Jallo GI. The Strata programmable valve for shunt-dependent hydrocephalus: The pediatric experience at a single institution. Childs Nerv Syst. 2006. 23: 297-303
2. Anderson RC, Walker ML, Viner JM, Kestle JR. Adjustment and malfunction of a programmable valve after exposure to toy magnets. J Neurosurg Pediatr. 2004. 101: 222-5
3. Fransen P, Dooms G, Thauvoy C. Safety of the adjustable pressure ventricular valve in magnetic resonance imaging: Problems and solutions. Neuroradiology. 1992. 34: 508-9
4. He Y, Murphy RK, Roland JL, Limbrick DD. Interactions between programmable shunt valves and the iPad 3 with Smart Cover. Childs Nerv Syst. 2013. 29: 531-3
5. . Magnetic Field Influences and Your Strata Valve [Pamphlet]. Medtronic. 2017. p.
6. Ozturk S, Cakin H, Kurtuldu H, Kocak O, Erol FS, Kaplan M. Smartphones and Programmable Shunts: Are These Indispensable Phones Safe and Smart?. World Neurosurg. 2017. 102: 518-25
7. Pierson MJ, Wehrmann D, Albers JA, El Tecle NE, Costa D, Elbabaa SK. Programmable shunt valve interactions with osseointegrated hearing devices. J Neurosurg Pediatr. 2017. 19: 384-90
8. Schneider T, Knauff U, Nitsch J, Firsching R. Electromagnetic field hazards involving adjustable shunt valves in hydrocephalus. J Neurosurg. 2002. 96: 331-4
9. Spader HS, Ratanaprasatporn L, Morrison JF, Grossberg JA, Cosgrove GR. Programmable shunts and headphones: Are they safe together?. J Neurosurg Pediatr. 2015. 16: 402-5
10. Strahle J, Collins K, Stetler WR, Smith BW, Garton T, Garton C. Effect of Amusement Park Rides on Programmable Shunt Valve Settings. Pediatr Neurosurg. 2013. 49: 21-3
11. Utsuki S, Shimizu S, Oka H, Suzuki S, Fujii K. Alteration of the Pressure Setting of a Codman-Hakim Programmable Valve by a Television. Neurol Med Chir. 2006. 46: 405-7
12. Retrieved September 22, 2017. from https://www.vocera.com/product/vocera-badge.
13. Xu H, Wang ZX, Liu F, Tan GW, Zhu HW, Chen DH. Programmable shunt valves for the treatment of hydrocephalus: A systemic review. Eur J Paediatr Neurol. 2013. 17: 454-61
14. Zuzak TJ, Balmer B, Schmidig D, Boltshauser E, Grotzer MA. Magnetic toys: Forbidden for pediatric patients with certain programmable shunt valves?. Childs Nerv Syst. 2008. 25: 161-4