- Department of Neurosurgery, Baylor College of Medicine/Texas Children's Hospital, Houston, Texas, USA
Correspondence Address:
Sandi Lam
Department of Neurosurgery, Baylor College of Medicine/Texas Children's Hospital, Houston, Texas, USA
DOI:10.4103/2152-7806.196919
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Visish M. Srinivasan, Loyola V. Gressot, Bradley S. Daniels, Jeremy Y. Jones, Andrew Jea, Sandi Lam. Management of intracerebral hemorrhage in pediatric neurosurgery. 28-Dec-2016;7:
How to cite this URL: Visish M. Srinivasan, Loyola V. Gressot, Bradley S. Daniels, Jeremy Y. Jones, Andrew Jea, Sandi Lam. Management of intracerebral hemorrhage in pediatric neurosurgery. 28-Dec-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/management-of-intracerebral-hemorrhage-in-pediatric-neurosurgery/
Keywords: Arteriovenous malformation, cavernoma, cavernous malformation, intracerebral hemorrhage, intracranial hemorrhage, neurovascular
INTRODUCTION
Pediatric stroke is a relatively rare occurrence, with an annual incidence of 1.2–13 cases per 100,000. Hemorrhagic strokes account for half of these cases.[
ILLUSTRATIVE CASES
Case 1
A 7-year-old girl presented with acute onset headache, aphasia, and right hemiparesis. She was found to have a large left temporal intracerebral hemorrhage (ICH) and underwent emergent decompressive craniectomy without direct evacuation of hematoma [
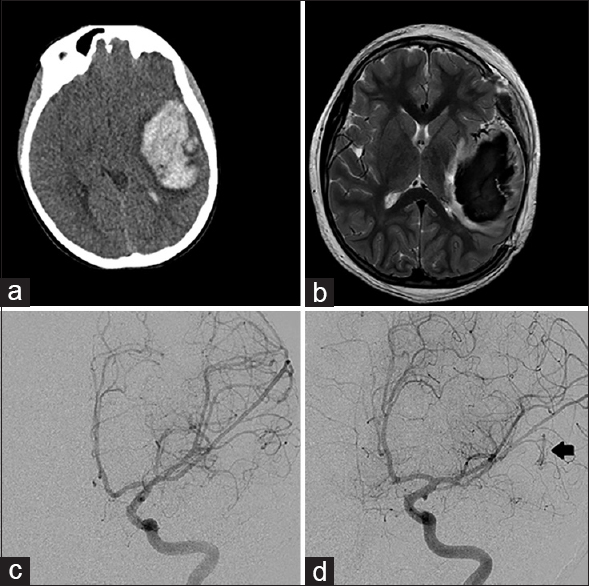
Figure 1
Left frontal hemorrhagic lesion from Case 1. (a) Computed tomography of the head at presentation showed a 9 × 4 × 4 cm hematoma with midline shift. (b) Magnetic resonance imaging brain, T2 sequence showed surrounding vessels but no definite arteriovenous malformation nidus around the large hematoma. (c) Left internal carotid artery digital subtraction angiogram at presentation, without evidence of vascular malformation. (d) Left internal carotid artery digital subtraction angiogram at 4 months post-hemorrhage reveals a left temporal Grade 1 arteriovenous malformation (arrow)
Case 2
A 9-year-old girl presented with acute onset of headache and obtundation. CT of the head demonstrated a large right cerebellar hemorrhage with tonsillar herniation, brainstem compression, and obstructive hydrocephalus [
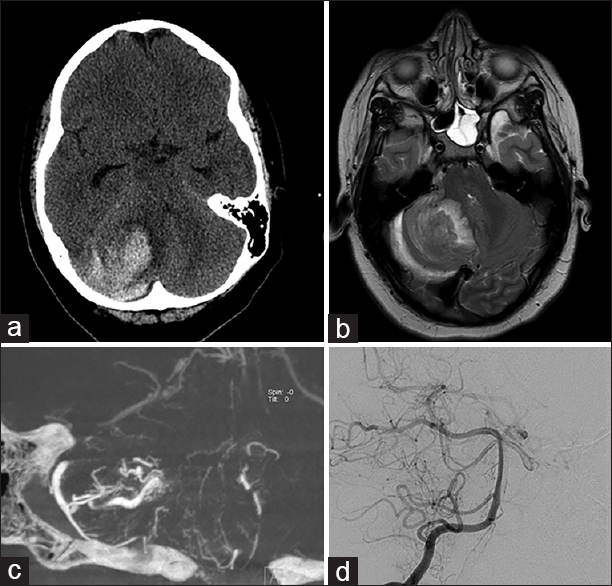
Figure 2
Right cerebellar hemorrhagic lesion from Case 2. (a) Computed tomography of the head at presentation showed a large hemorrhage of the right cerebellar hemisphere with displacement of the fourth ventricle. (b) Magnetic resonance imaging brain, T2 sequence showed perilesional edema and some associated vessels. (c) Right vertebral artery injection DynaCT, coronal view, showed an arteriovenous malformation with feeders from the right anterior and posterior cerebellar arteries. (d) Postoperative angiogram, AP projection, showed no residual malformation
Case 3
A 14-year-old girl presented with acute onset of headache, nausea, and vomiting for 1 day. She had similar but mild symptoms 2 weeks prior. She had a generalized seizure upon arrival to the hospital. CT of the head revealed a large 5 cm left frontal hemorrhage with mixed density and significant mass effect with exuberant perilesional edema [
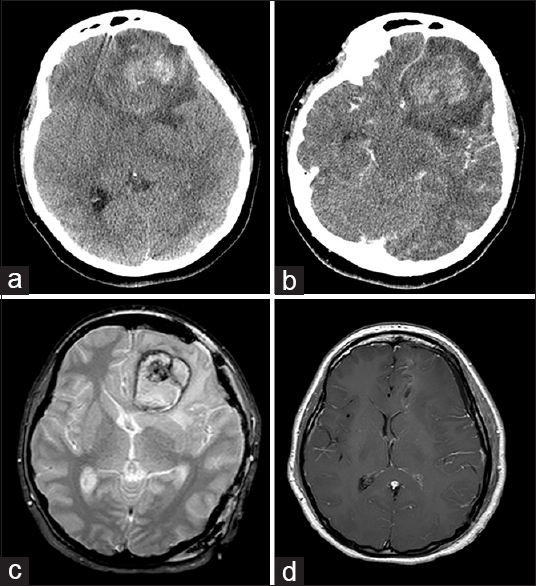
Figure 3
Left frontal hemorrhage from Case 3. (a) Computed tomography of the head showed a large left frontal intracerebral hemorrhage with subfalcine shift and perilesional edema. (b) Computed tomography with contrast showed partial enhancement of the lesion without any vascular feeders. (c) Magnetic resonance imaging brain, gradient echo sequence, showed a central lesion with surrounding hemorrhage, suggestive of cavernous malformation. (d) Postoperative magnetic resonance imaging brain with contrast showed complete resection of the lesion and hematoma, with resolution of mass effect
MANAGEMENT
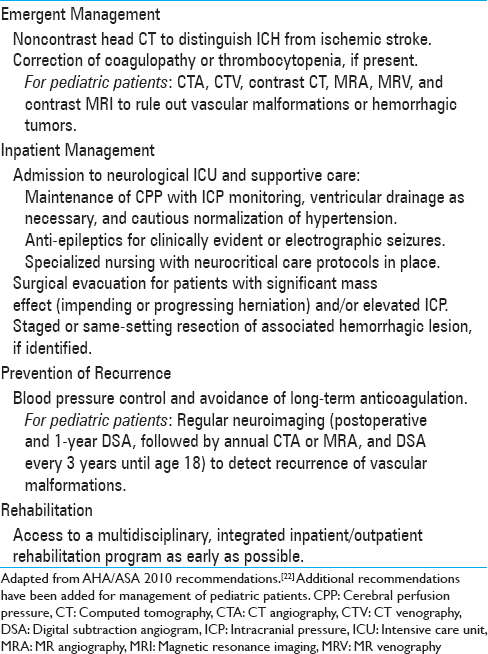
Upon initial diagnosis of intracerebral hemorrhage on noncontrast CT, workup and treatment should be initiated without delay. The guidelines for the treatment of spontaneous ICH were last updated in 2010; these recommendations also apply to pediatric patients [
SURGICAL NUANCES
Several surgical options exist for managing acute ICH requiring intervention. First and foremost are the evaluation and management of “ABCs:” Airway, breathing, and circulation. If high intracranial pressure is present, it must be addressed with external ventricular drainage, evacuation of the hematoma, and/or decompressive craniectomy with expansile duraplasty depending on the clinical situation and the location of the hemorrhage. If there is suspicion of underlying AVM, limited evacuation of the hematoma is advised only if necessary in cases of mass effect because aggressive evacuation of the hematoma may precipitate AVM re-rupture. The thrombus cap over the rupture site can be tenuous.[
In hemorrhages seemingly admixed with eloquent brain tissue, our experience supports a large craniectomy with expansile duraplasty in cases of significant cerebral edema, without surgical destruction of eloquent areas. This allows for the possibility of recovery of function. Direct removal of the hematoma and the vascular malformation at initial surgery may not be possible due to high ICP and incomplete workup due to clinical instability, and thus, incomplete appreciation for the anatomy and localization of the lesion, as described in Case 1. Craniectomy or minimal evacuation of hematoma, followed by a more lesion-tailored approach with the support of diagnostic imaging, may be prudent in these cases. In contrast to ruptured aneurysms that have a high short-term risk of re-rupture, thus warranting urgent securement, ruptured AVMs and cavernomas have relatively low acute re-rupture rates, which allow slightly more conservative management.[
ARTERIOVENOUS MALFORMATIONS
AVMs are the most common cause of ICH in children. They carry an annual risk of hemorrhage of approximately 3.2%, a 5–10% mortality rate, and a 50% risk of neurological morbidity.[
Postoperative surveillance of AVM
AVMs in children are fraught with a high recurrence rate (as high as 14%),[
CAVERNOUS MALFORMATIONS
CMs represent 20–25% of spontaneous ICH in children.[
CONCLUSION
Intracerebral hemorrhage in children requires special consideration in management compared to the same in adults. The rate of associated vascular malformations, including but not limited to AVMs and CMs, is high. These should be considered during initial evaluation, hematoma management, and surgical management of the offending lesion.[
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abla AA, Rutledge WC, Seymour ZA, Guo D, Kim H, Gupta N. A treatment paradigm for high-grade brain arteriovenous malformations: Volume-staged radiosurgical downgrading followed by microsurgical resection. J Neurosurg. 2015. 122: 419-32
2. Al-Holou WN, O’Lynnger TM, Pandey AS, Gemmete JJ, Thompson BG, Muraszko KM. Natural history and imaging prevalence of cavernous malformations in children and young adults. J Neurosurg Pediatr. 2012. 9: 198-205
3. Bilginer B, Narin F, Hanalioglu S, Oguz KK, Soylemezoglu F, Akalan N. Cavernous malformations of the central nervous system (CNS) in children: Clinico-radiological features and management outcomes of 36 cases. Childs Nerv Syst. 2014. 30: 1355-66
4. Blauwblomme T, Bourgeois M, Meyer P, Puget S, Di Rocco F, Boddaert N. Long-term outcome of 106 consecutive pediatric ruptured brain arteriovenous malformations after combined treatment. Stroke. 2014. 45: 1664-71
5. Clatterbuck RE, Moriarity JL, Elmaci I, Lee RR, Breiter SN, Rigamonti D. Dynamic nature of cavernous malformations: A prospective magnetic resonance imaging study with volumetric analysis. J Neurosurg. 2000. 93: 981-6
6. de Champfleur NM, Langlois C, Ankenbrandt WJ, Le Bars E, Leroy MA, Duffau H. Magnetic resonance imaging evaluation of cerebral cavernous malformations with susceptibility-weighted imaging. Neurosurgery. 2011. 68: 641-
7. Greenberg MS.editorsHandbook of Neurosurgery. Tampa: Greenberg Graphics Inc; 2010. p.
8. Gross BA, Du R. Natural history of cerebral arteriovenous malformations: A meta-analysis. J Neurosurg. 2013. 118: 437-43
9. Gross BA, Du R, Orbach DB, Scott RM, Smith ER. The natural history of cerebral cavernous malformations in children. J Neurosurg Pediatr. 2015. p. 1-6
10. Hoh BL, Ogilvy CS, Butler WE, Loeffler JS, Putman CM, Chapman PH. Multimodality treatment of nongalenic arteriovenous malformations in pediatric patients. Neurosurgery. 2000. 47: 346-
11. Ide C, De Coene B, Baudrez V. MR features of cavernous angioma. JBR-BTR. 2000. 83: 320-
12. Jordan LC, Hillis AE. Hemorrhagic stroke in children. Pediatr Neurol. 2007. 36: 73-80
13. Kano H, Kondziolka D, Flickinger JC, Yang HC, Flannery TJ, Awan NR. Stereotactic radiosurgery for arteriovenous malformations, part 2: Management of pediatric patients. J Neurosurg Pediatr. 2012. 9: 1-10
14. Kim H, Abla AA, Nelson J, McCulloch CE, Bervini D, Morgan MK. Validation of the supplemented Spetzler-Martin grading system for brain arteriovenous malformations in a multicenter cohort of 1009 surgical patients. Neurosurgery. 2015. 76: 25-31
15. Klimo P, Rao G, Brockmeyer D. Pediatric arteriovenous malformations: A 15-year experience with an emphasis on residual and recurrent lesions. Childs Nerv Syst. 2007. 23: 31-7
16. Lawton MT.editorsSeven AVMs: Tenets and Techniques for Resection. New York: Thieme; 2014. p.
17. Lawton MT, Kim H, McCulloch CE, Mikhak B, Young WL. A supplementary grading scale for selecting patients with brain arteriovenous malformations for surgery. Neurosurgery. 2010. 66: 702-13
18. Lin N, Smith ER, Scott RM, Orbach DB. Safety of neuroangiography and embolization in children: Complication analysis of 697 consecutive procedures in 394 patients. J Neurosurg Pediatr. 2015. 16: 432-8
19. Liu AC, Segaren N, Cox TS, Hayward RD, Chong WK, Ganesan V. Is there a role for magnetic resonance imaging in the evaluation of non-traumatic intraparenchymal haemorrhage in children?. Pediatr Radiol. 2006. 36: 940-6
20. Meretoja A, Strbian D, Putaala J, Curtze S, Haapaniemi E, Mustanoja S. SMASH-U: A proposal for etiologic classification of intracerebral hemorrhage. Stroke. 2012. 43: 2592-7
21. Meyer-Heim AD, Boltshauser E. Spontaneous intracranial haemorrhage in children: Aetiology, presentation and outcome. Brain Dev. 2003. 25: 416-21
22. Morgenstern LB, Hemphill JC, Anderson C, Becker K, Broderick JP, Connolly ES. Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010. 41: 2108-29
23. Morgenstern PF, Hoffman CE, Kocharian G, Singh R, Stieg PE, Souweidane MM. Postoperative imaging for detection of recurrent arteriovenous malformations in children. J Neurosurg Pediatr. 2015. p. 1-7
24. Ogilvy CS, Heros RC, Ojemann RG, New PF. Angiographically occult arteriovenous malformations. J Neurosurg. 1988. 69: 350-5
25. Osborn AG.editorsOsborn's Brain: Imaging, Pathology, and Anatomy. Salt Lake City: Amirsys Publishing, Inc; 2013. p.
26. Srinivasan VM, Schafer S, Ghali MG, Arthur A, Duckworth EA. Cone-beam CT angiography (Dyna CT) for intraoperative localization of cerebral arteriovenous malformations. J Neurointerv Surg. 2016. 8: 69-74
27. Tsitsopoulos P, Andrew J, Harrison MJ. Occult cerebral arteriovenous malformations. J Neurol Neurosurg Psychiatry. 1987. 50: 218-20
28. Tsze DS, Valente JH. Pediatric stroke: A review. Emerg Med Int 2011. 2011. p. 734506-
29. Wardlaw JM, Keir SL, Seymour J, Lewis S, Sandercock PA, Dennis MS. What is the best imaging strategy for acute stroke?. Health Technol Assess. 2004. 8: iii-ix-x
30. Zabramski JM, Wascher TM, Spetzler RF, Johnson B, Golfinos J, Drayer BP. The natural history of familial cavernous malformations: Results of an ongoing study. J Neurosurg. 1994. 80: 422-32