- Department of Neurosurgery, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan.
- Department of Clinical Chemistry and Laboratory Medicine, Kyushu University Hospital, Fukuoka, Japan.
- Department of Health Sciences, Division of Medical Technology, Fukuoka, Japan.
- Department of Neurology, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan.
- Department of Neurosurgery, Hachisuga Hospital, Fukuoka, Japan.
Correspondence Address:
Takafumi Shimogawa, Department of Neurosurgery, Kyushu University, Fukuoka, Japan.
DOI:10.25259/SNI_1164_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Takafumi Shimogawa1, Ayumi Sakata2,3, Eriko Watanabe2, Nobutaka Mukae1, Hiroshi Shigeto3,4, Takahiko Mukaino4, Toshiki Okadome4, Takahiro Yamaguchi4, Koji Yoshimoto1, Takato Morioka5. Mandibular and chin electrodes as a supplemental recording for detection of epileptiform discharges in mesial temporal lobe epilepsy. 02-Jun-2023;14:189
How to cite this URL: Takafumi Shimogawa1, Ayumi Sakata2,3, Eriko Watanabe2, Nobutaka Mukae1, Hiroshi Shigeto3,4, Takahiko Mukaino4, Toshiki Okadome4, Takahiro Yamaguchi4, Koji Yoshimoto1, Takato Morioka5. Mandibular and chin electrodes as a supplemental recording for detection of epileptiform discharges in mesial temporal lobe epilepsy. 02-Jun-2023;14:189. Available from: https://surgicalneurologyint.com/surgicalint-articles/12347/
Abstract
Background: We previously demonstrated the usefulness of periorbital electrodes in supplemental recording to detect epileptiform discharges in patients with mesial temporal lobe epilepsy (MTLE). However, eye movement may disturb periorbital electrode recording. To overcome this, we developed mandibular (MA) and chin (CH) electrodes and examined whether these electrodes could detect hippocampal epileptiform discharges.
Methods: This study included a patient with MTLE, who underwent insertion of bilateral hippocampal depth electrodes and video-electroencephalographic (EEG) monitoring with simultaneous recordings of extra- and intracranial EEG as part of a presurgical evaluation. We examined 100 consecutive interictal epileptiform discharges (IEDs) recorded from the hippocampus and two ictal discharges. We compared these IEDs from intracranial electrodes with those from extracranial electrodes such as MA and CH electrodes in addition to F7/8 and A1/2 of international EEG 10-20 system, T1/2 of Silverman, and periorbital electrodes. We analyzed the number, rate of laterality concordance, and mean amplitude of IEDs detected in extracranial EEG monitoring and characteristics of IEDs on the MA and CH electrodes.
Results: The MA and CH electrodes had nearly the same detection rate of hippocampal IEDs from other extracranial electrodes without contamination by eye movement. Three IEDs, not detected by A1/2 and T1/2, could be detected using the MA and CH electrodes. In two ictal events, the MA and CH electrodes detected the ictal discharges from the hippocampal onset as well as other extracranial electrodes.
Conclusion: The MA and CH electrodes could detect hippocampal epileptiform discharges as well as A1/A2, T1/T2, and peri-orbital electrodes. These electrodes could serve as supplementary recording tools for detecting epileptiform discharges in MTLE.
Keywords: Chin electrode, Electroencephalography, Epileptiform discharge, Mandibular electrode, Mesial temporal lobe epilepsy
INTRODUCTION
The mesial temporal lobe is a key structure that is involved in epileptiform discharges, particularly the hippocampus. However, extracranial detection of electroencephalographic (EEG) activity from the mesial temporal lobe is sometimes difficult to detect with standard surface electrodes placed according to the international 10–20 EEG system, such as F7/F8 and A1/A2, because the conduction of deep brain activity is easily attenuated by the skull.[
MATERIALS AND METHODS
Patient information
A 51-year-old right-handed man was admitted to our institution. The patient developed focal impaired awareness seizures 2 years before admission, which became resistant to optimal doses of anti-seizure medications (ASMs) including valproate, levetiracetam, and lacosamide. EEG demonstrated independent interictal epileptiform discharges (IEDs) and ictal discharges in the bilateral temporal regions. Magnetic resonance imaging was normal. 18Fluorodeoxyglucose-positron emission tomography showed a slightly hypometabolic region in the right medial and lateral temporal lobe. The Wada test suggested left hemispheric dominance for language but bilateral for memory. He underwent stereotactic insertion of bilateral hippocampal depth electrodes with four contacts (electrodes no. 1–4 and 7–10) at 5 mm intervals and two contacts (electrodes no. 5–6 and 11–12) at 10 mm intervals [
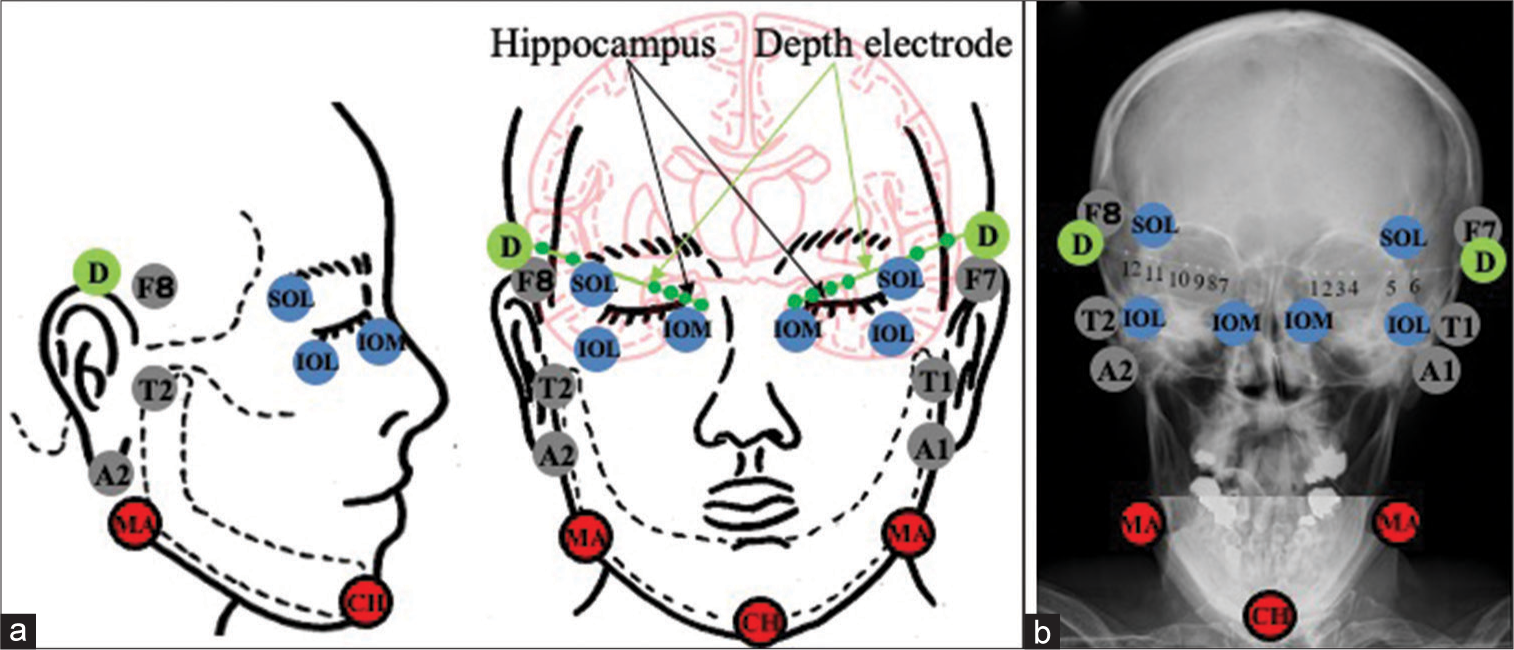
Figure 1:
Schematic drawing (a) and plain craniogram (b) showing the anatomical relationship between the standard extracranial anterior temporal electrodes placed according to the international EEG 10-20 system (F7/8 and A1/A2), T1/T2 of Silverman, periorbital electrodes (supraorbital lateral, infraorbital lateral and infraorbital medial), and the mandibular and chin electrodes, and bilateral hippocampal depth electrodes (D) with six contacts (1–6 on the left side and 7–12 on the right side). SOL: Supraorbital lateral, IOL: Infraorbital lateral, ÏOM: Infraorbital medial, MA: Mandibular, CH: Chin.
This study was approved by the Ethics Committee of our institution (No. 2021-370). The patient provided written informed consent.
Simultaneous extra- and intracranial EEG recording
For extracranial EEG recording, disc electrodes were placed over the scalp according to the international 10–20 EEG system. Moreover, T1/T2 electrodes[
Extra- and intracranial EEG data analysis
We examined IEDs detected in extracranial EEG monitoring, including those from the MA and CH electrodes, along with 100 consecutive IEDs detected from intracranial EEG monitoring. The IED amplitude was calculated by measuring the difference between the positive peak and the negative peak. We analyzed the number, the rate of laterality concordance, and mean amplitude of IEDs detected in extracranial EEG monitoring. The relationship between the appearance of IEDs on extracranial EEG and the appearance and the amplitude of the corresponding IEDs on intracranial EEG was analyzed retrospectively by two board-certified electroencephalographers (T.S. and A.S.) who were blinded to the clinical data. No differences in the electroencephalographers’ interpretations were noted in the independent assessment. A Pz electrode was used as the reference electrode for the amplitude measurement. Amplification sensitivity and filter settings were changed when necessary. The amplitude from the peak to baseline of the IED was measured using a digital scaling system (Nihon Kohden Tokyo, Japan).
RESULTS
The number of the extracranial IEDs recorded concurrently with 100 consecutive hippocampal IEDs
We counted the number of the IEDs recorded from each extracranial EEG electrode, including F7/8, A1/2, T1/2, SOL (Left [L]/Right [R]), IOM (L/R), IOL (L/R), and MA (L/R)+CH, corresponding to 100 consecutive hippocampal IEDs [
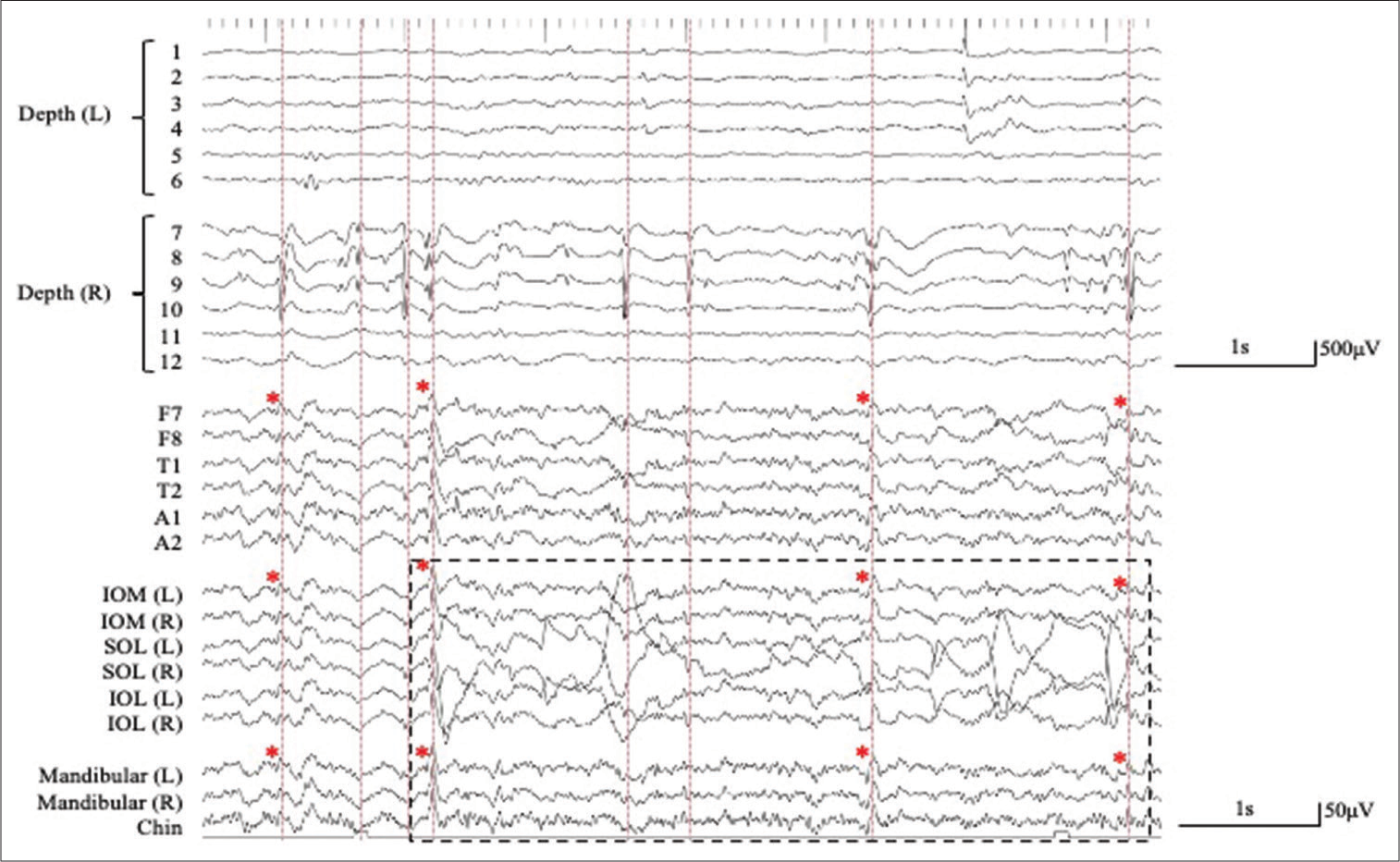
Figure 2:
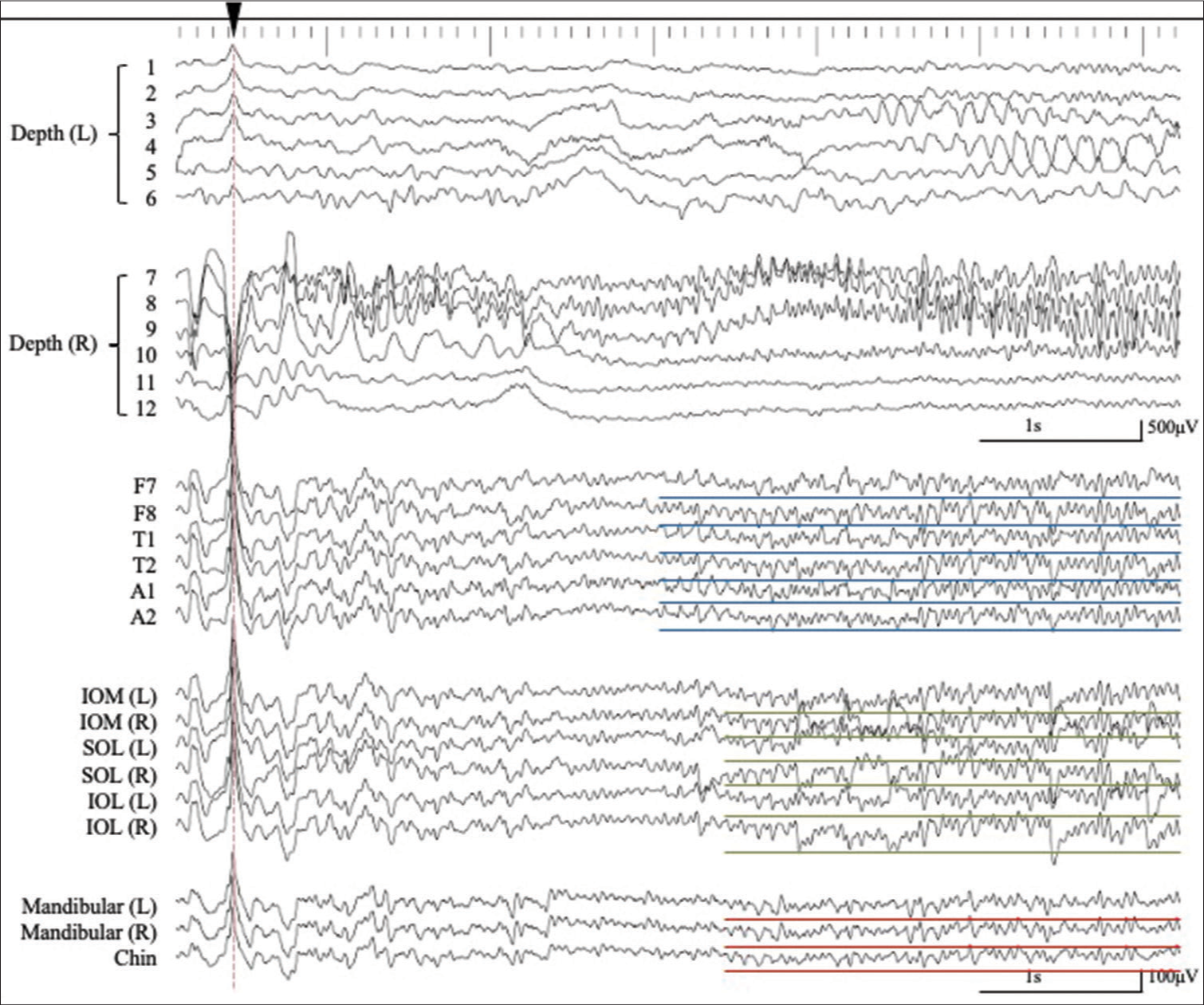
Simultaneous extra- and intracranial (bilateral hippocampal depth electrodes with six contacts; 1–6 on the left (L) side and 7–12 on the right (R) side) EEG recording. The interictal epileptiform discharges (IEDs) detected in extracranial EEG monitoring (lower traces) correspond to IEDs detected in the bilateral hippocampus as intra-cranial EEG monitoring (upper traces) (red dashed lines). A number of the depth electrodes are indicated on Figure 1. (Pz, averaged reference). The IEDs detected on the extracranial recording are shown with red asterisks. The artifact associated with eye movement is recorded by the periorbital electrodes, but not by the mandibular + chin electrodes (black dashed square). SOL: Supraorbital lateral, IOL: Infraorbital lateral, IOM: Infraorbital medial.
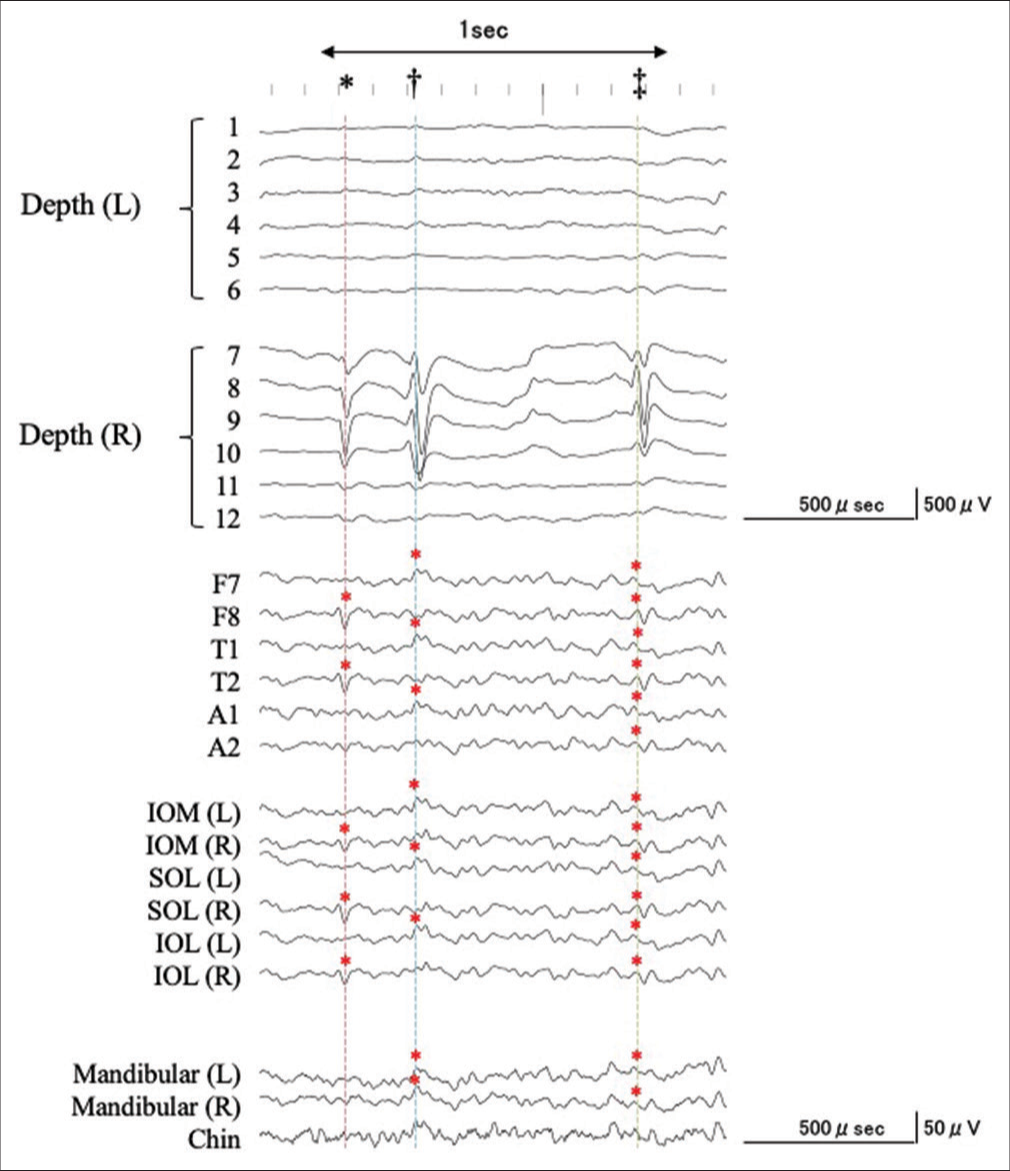
Figure 3:
An example of the simultaneous intra- and extracranial EEG recording with expanded time-scale. See details in the text. Corresponding to a negative component of an IED recorded from the right hippocampus (7–9), negative IEDs were recorded from F7, T1, A1, IOM (L), SOL (L), IOL (L), MA (L/R), and CH (dagger, blue dashed line). Corresponding with the predominantly positive component of the IED from the right hippocampus (7–9), a corresponding negative IED was recorded at F7/8, T1/2, A1/2, IOM (L/R), SOL (L/R), IOL (L/R), MA (L/R), and CH (double dagger, green dashed line), whereas the predominantly positive components were detected at F8, T2, IOM (R), SOL (R), and IOL (R). However, IEDs were not detected from the MA and CH electrodes (asterisk, red dashed line).
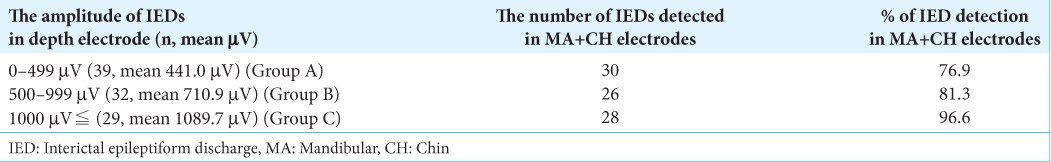
The MA and CH electrodes had the almost same detection rate as the hippocampal IEDs obtained from A1/2 and T1/2, that is, out of 100 intracranial IEDs, 41 IEDs were detected by electrodes at F7/8, 49 at A1/2, 53 at T1/2, 53 at SOL, 60 at IOM, 47 at IOL, and 51 at MA+CH (11 by MA and 40 by CH) [
There were three hippocampal IEDs with predominantly positive components not detected by A1/2 and T1/2, but they were slightly by the MA and CH electrodes [
The detection rate of IED potentials by MA and CH electrodes compared with intracranial EEG
We divided the amplitude of IEDs detected in hippocampal EEG into three groups: 0–499 μV (39 IEDs, mean 441.0 μV) (Group A), 500–999 μV (32 IEDs, mean 710.9 μV) (Group B), and over 1000 μV (29 IEDs, mean 1089.7 μV) (Group C). Extracranial EEG recordings demonstrated that 30 IEDs in Group A, 26 in Group B, and 28 in Group C were detected [
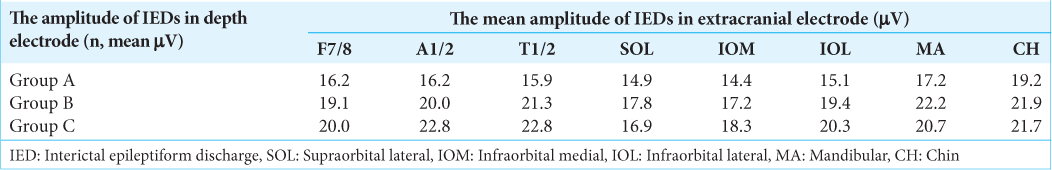
The mean amplitude of the IEDs in extracranial EEG compared with IED potentials in intracranial EEG
The relationship between the mean amplitudes of the IEDs in intra- and extracranial EEGs are described on
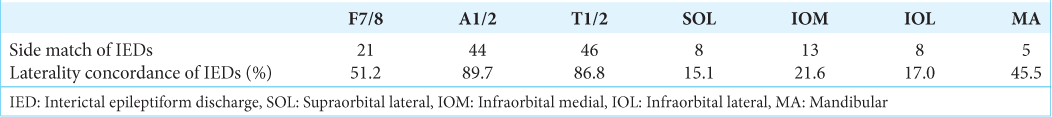
The laterality concordance between extra- and intracranial IEDs
The IEDs detected on the extracranial EEG were divided into two groups: those that matched the side of the intracranial IEDs and those that did not [
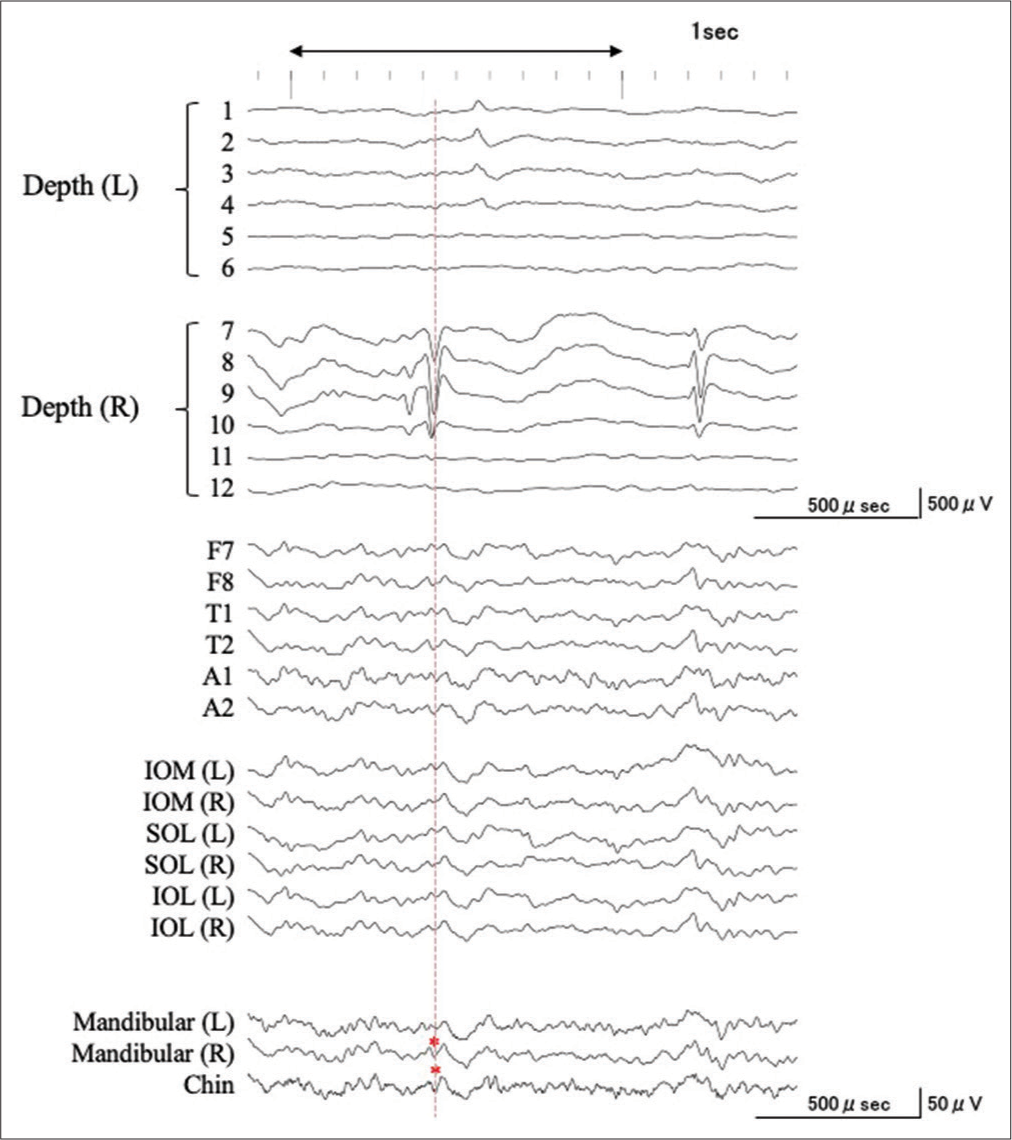
Simultaneous ictal extra- and intracranial EEG recording
Two ictal events were recorded. Corresponding to the positive wave at the ictal onset from the right hippocampus, a negative potential following desynchronization with amplitude attenuation was recorded at all electrodes of the extracranial EEG [
Figure 5:
Simultaneous ictal extra- and intra-cranial EEG recording. Corresponding to the positive wave with the amplitude of more than 1500 μV at the ictal onset from the right hippocampus, a negative potential is recorded by all electrodes of the extracranial EEG (black arrowhead and red dotted line). Subsequent ictal discharges are recorded at F7/8, A1/2, T1/2 (blue lines), and 0.4 s later, also recorded at SOL, IOM, IOL (green lines), and MA+CH electrodes (red lines). SOL: Supraorbital lateral, IOM: Infraorbital medial, IOL: Infraorbital lateral, MA: Mandibular, CH: Chin.
DISCUSSION
Two major reasons for the limited sensitivity of the extracranial recording to IEDs from the mesial temporal lobe are due to the rapid decay of the hippocampal IEDs due to the volume conduction and smearing effect of the skull, as we demonstrated in our previous reports.[
However, the amplitude of extracranial IEDs from MA and CH electrodes was not generally proportional to the amplitude of the intracranial IED, whereas the higher the amplitude of the intracranial IED, the higher the amplitude of the extracranial IED at F7/8, A1/2, and T1/2. These findings suggest that the relationship between the intra- and extracranially detected IEDs is not straightforward[
In terms of the ictal discharges, the MA and CH electrodes also could detect ictal events from the hippocampal onset, the same as other extracranial electrodes. Our previous report[
The drawback of the MA and CH electrodes is that the rate of laterality concordance was inferior to those of the standard temporal surface electrodes and T1/2, though superior to that of the periorbital electrodes. This is again thought to be due to the fact that the MA and CH electrodes are far from the mesial temporal lobe. It is also expected that the diagnosis of laterality will be difficult when the IEDs originating from the hippocampus located near the midline are recorded from below.
The present study had several limitations. First, this study was performed in only one patient with MTLE. MTLE patients undergo epilepsy surgery without invasive examination in our institution and we rarely have the chance to perform simultaneous intra- and extracranial recordings.[
CONCLUSION
The MA and CH electrodes could detect IEDs in MTLE and were not inferior to A1/A2, T1/T2, and periorbital electrodes. These electrodes could serve as supplementary recording tools for detecting epileptiform discharges in MTLE.
Declaration of patient consent
The authors certify that they have obtained appropriate patient consent.
Financial support and sponsorship
This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS) (JP21K17456 to T.S.).
Conflicts of Interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
We thank Kazuyuki Matsuo and Masako Fujise as well as their colleagues at Kyushu University Hospital for supporting our study.
References
1. Bayram AK, Spencer DD, Alkawadri R. Tongue as a wire? Glossokinetic artifact and insights from intracranial EEG. J Clin Neurophysiol. 2022. 39: 481-5
2. Hashiguchi K, Morioka T, Yoshida F, Miyagi Y, Nagata S, Sakata A. Correlation between scalp-recorded electroencephalographic and electrocorticographic activities during ictal period. Seizure. 2007. 16: 238-47
3. Kanner AM, Parra J, Gil-Nagel A, Soto A, Leurgans S, Iriarte J. The localizing yield of sphenoidal and anterior temporal electrodes in ictal recordings: A comparison study. Epilepsia. 2002. 43: 1189-96
4. Kissani N, Alarcon G, Dad M, Binnie CD, Polkey CE. Sensitivity of recordings at sphenoidal electrode site for detecting seizure onset: Evidence from scalp, superficial and deep foramen ovale recordings. Clin Neurophysiol. 2001. 112: 232-40
5. Krauss GL, Lesser RP, Fisher RS, Arroyo S Anterior. “cheek” electrodes are comparable to sphenoidal electrodes for the identification of ictal activity. Electroencephalogr Clin Neurophysiol. 1992. 83: 333-8
6. Lee S, Wu S, Tao JX, Rose S, Warnke PC, Issa NP. Manifestation of hippocampal interictal discharges on clinical scalp EEG recordings. J Clin Neurophysiol. 2021. 40: 144-50
7. Mukae N, Shimogawa T, Sakata A, Uehara T, Shigeto H, Yoshimoto K. Reflection of the ictal electrocorticographic discharges confined to the medial temporal lobe to the scalp-recorded electroencephalogram. Clin EEG Neurosci. 2021. 54: 173-8
8. Sakai Y, Nagano H, Sakata A, Kinoshita S, Hamasaki N, Shima F. Localization of epileptogenic zone in temporal lobe epilepsy by ictal scalp EEG. Seizure. 2002. 11: 163-8
9. Sakata A, Mukae N, Morioka T, Tanaka S, Shimogawa T, Shigeto H. Simultaneous electroencephalographic and electocorticographic recordings of lateralized periodic discharges in temporal lobe epilepsy. Clin EEG Neurosci. 2022. 53: 61-9
10. Shigeto H, Sakata A, Morioka T, Takase K, Hagiwara K, Kamada T. Peri-orbital electrodes as a supplemental recording for detection of ictal discharges in medial temporal lobe epilepsy. Neurol Asia. 2011. 16: 303-7
11. Silverman D. The anterior temporal electrode and the ten-twenty system. Electroencephalogr Clin Neurophysiol. 1960. 12: 735-7