- Department of Neurological Surgery, University of California, San Diego, La Jolla,
- Department of Neurological Surgery, University of California, Irvine, California, United States.
Correspondence Address:
Joseph Anthony Osorio
Department of Neurological Surgery, University of California, San Diego, La Jolla,
DOI:10.25259/SNI_514_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Luis Diaz-Aguilar1, Usman Khan1, Ronald Sahyouni1, Nolan James Brown2, Scott Olson1, Joseph Anthony Osorio1. Metastatic epidural spinal column compression due to pancreatic ductal adenocarcinoma causing subacute Cauda equina syndrome: A case report. 05-Sep-2020;11:279
How to cite this URL: Luis Diaz-Aguilar1, Usman Khan1, Ronald Sahyouni1, Nolan James Brown2, Scott Olson1, Joseph Anthony Osorio1. Metastatic epidural spinal column compression due to pancreatic ductal adenocarcinoma causing subacute Cauda equina syndrome: A case report. 05-Sep-2020;11:279. Available from: https://surgicalneurologyint.com/surgicalint-articles/10238/
Abstract
Background: Pancreatic ductal adenocarcinoma (PDAC) is the most common pancreatic malignancy, which rarely metastasizes to the spine.
Case Description: Here, we present a lytic lumbar metastatic PDAC resulting in severe epidural spinal cord compression (ESCC) with instability. The lesion required preoperative particle embolization, surgical decompression, and fusion.
Conclusion: This case report shows that PDAC may metastasize to the lumbar spine requiring routine decompression with fusion.
Keywords: Cauda equina syndrome, Decompression, Instrumentation, Pancreatic ductal adenocarcinoma, Spinal metastasis
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is a rare cause of metastatic disease to the spine.[
CASE REPORT
A 60-year-old female presented with shortness of breath, ascites, and a subacute progressive cauda equina syndrome and urinary incontinence attributed to a metastatic epidural spinal cord metastasis. CT/MR both documented destructive metastatic lesion of the L4 vertebral body. On the MR/CT studies, there was accompanying L4 extraosseous extension into the spinal canal, spinous process, and left paraspinal musculature (7.8 × 7.1 cm), and another L3 posterior vertebral body lesion also with extension into the spinal canal (2.1 × 2.0 cm) [
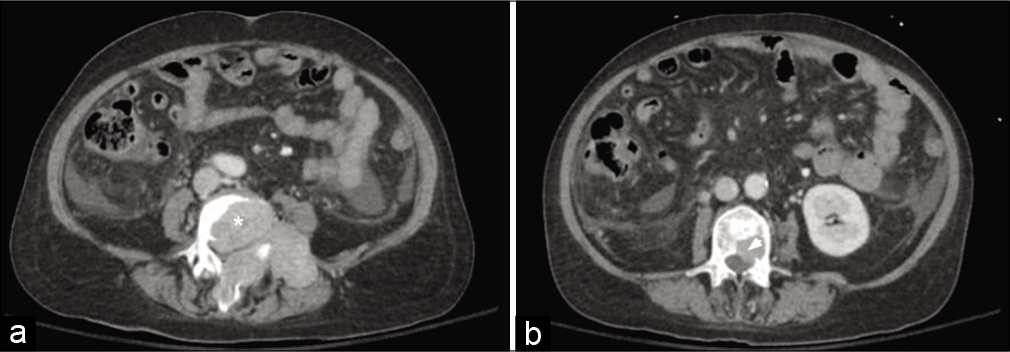
Figure 1:
Axial computed tomography abdomen and pelvis demonstrating L4 vertebral body destructive metastases (*) with extraosseous extension into the spinal canal, spinous process, and left paraspinal musculature measuring 7.8 × 7.1 cm (a) and L3 posterior vertebral body metastasis (arrow head) with intraosseous extension into the spinal canal measuring 2.1 × 2.0 cm (b).
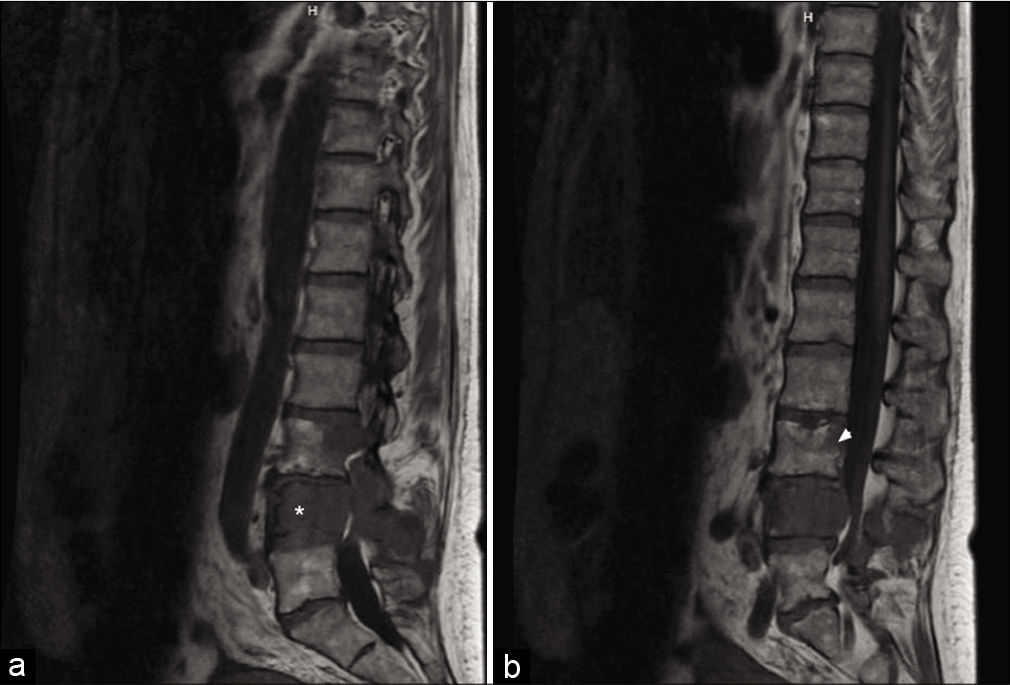
Figure 2:
Sagittal magnetic resonance imaging of the lumbar spine demonstrating near-complete marrow replacement of the L4 vertebral body (*) with expansile, locally destructive soft tissue with extension into the left posterior elements and spinous process (a). Associated extra cortical extension of disease with circumferential encasement of the epidural space resulting in extremely severe spinal canal stenosis with compression of the cauda equina nerve roots. There is also replacement of the posterior aspect of the L3 vertebral body (arrow head) and associated 20 percent posterior pathological compression fracture deformity (b). Frank extra cortical disease extension at this level results in moderate spinal canal narrowing with asymmetric effacement of the left lateral recess and compression of the traversing left L4 nerve roots.
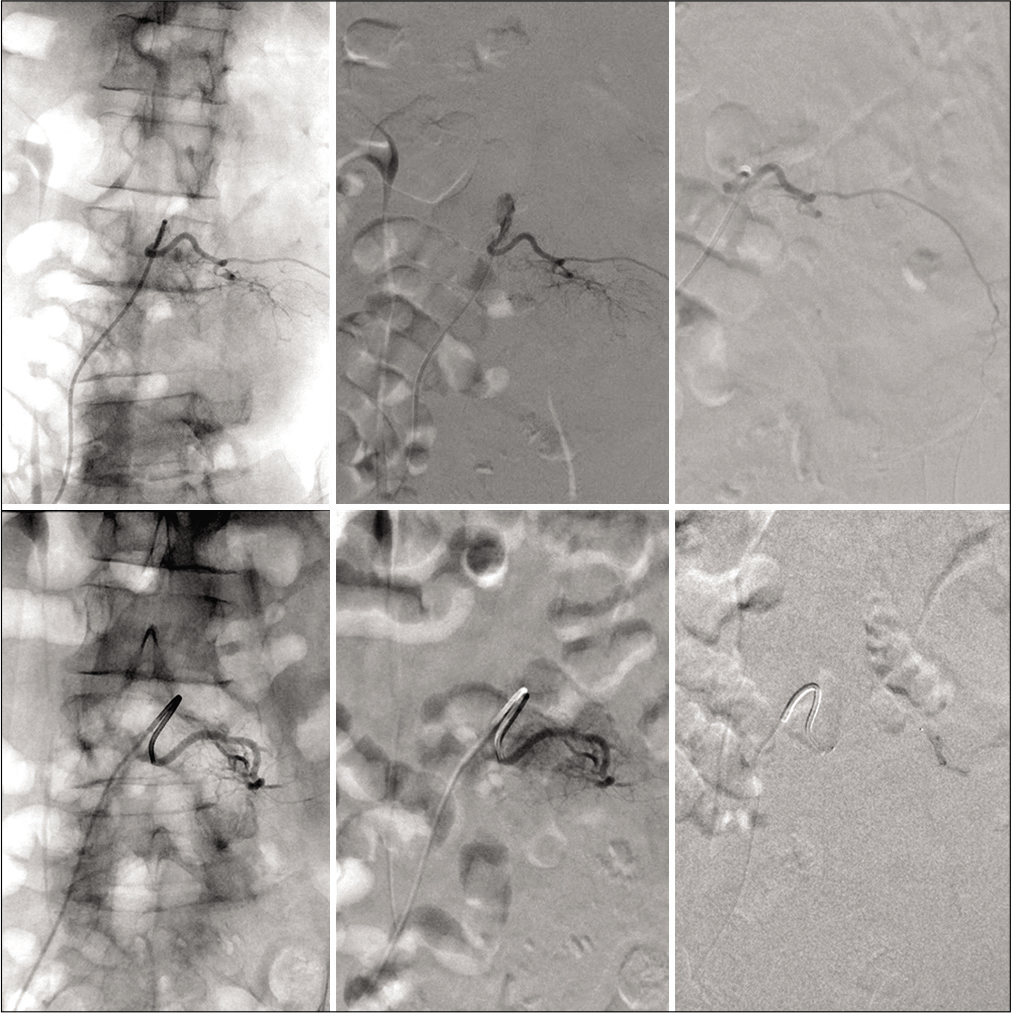
Figure 4:
Top row: Source AP fluoroscopy of left L3 radicular superselective angiography (left), digital subtraction angiography of preembolization tumor supply (middle), postembolization irrigation with obliteration of flow (right). Bottom row: similar representation of the left L4 superselective angiography with near total obliteration of supply.
Figure 6:
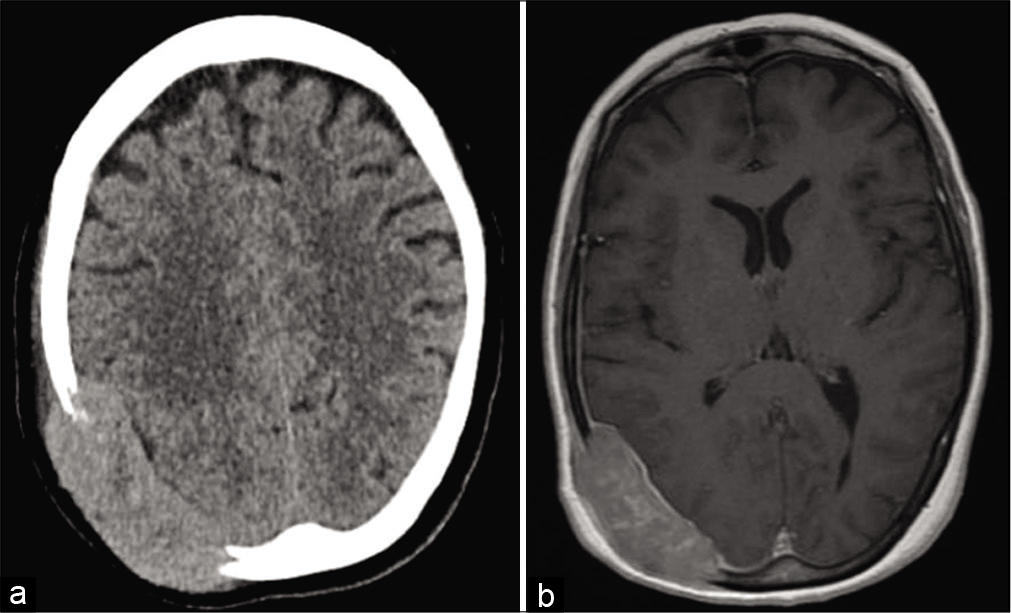
Computed tomography head demonstrating a large expansile transcalvarial lesion centered at the right occipital convexity measuring approximately 7.1 × 2.3 cm transaxially (a) with resultant sulcal effacement of the subject temporal, parietal, and occipital lobes and expansion into the adjacent scalp soft tissues. Magnetic resonance imaging of the mass is also demonstrated (b).
DISCUSSION
Pancreatic cancer is the seventh leading cause of cancer- related deaths worldwide, with a 5-year survival rate of <5%.[
The instability conferred by this patient’s lytic lesions at L3 and L4 was an independent indication for mechanical stabilization or cement augmentation. We prefer resection and stabilization through a posterolateral approach for maximum safe resection and stabilization. Cytoreduction also afforded the opportunity to perform molecular profiling and next-generation sequencing on tumor samples to tailor further therapies. In this case, the patient qualified for immunotherapy and EGFR/VEGF biologics. Preoperative embolization with polyvinyl alcohol (PVA) mitigated the risk of tumor hemorrhage and transfusion.
Treatment options for spinal metastatic disease due to PDAC
Although a number of cases of PDAC metastasizing to the skeletal system have been reported, relatively few studies specifically report metastases to the lumbar spine resulting in canal compression; only one appeared to undergo surgical intervention. One study identified seven patients from a database of 323 PDAC patients (2.2%) with skeletal metastases.[
CONCLUSION
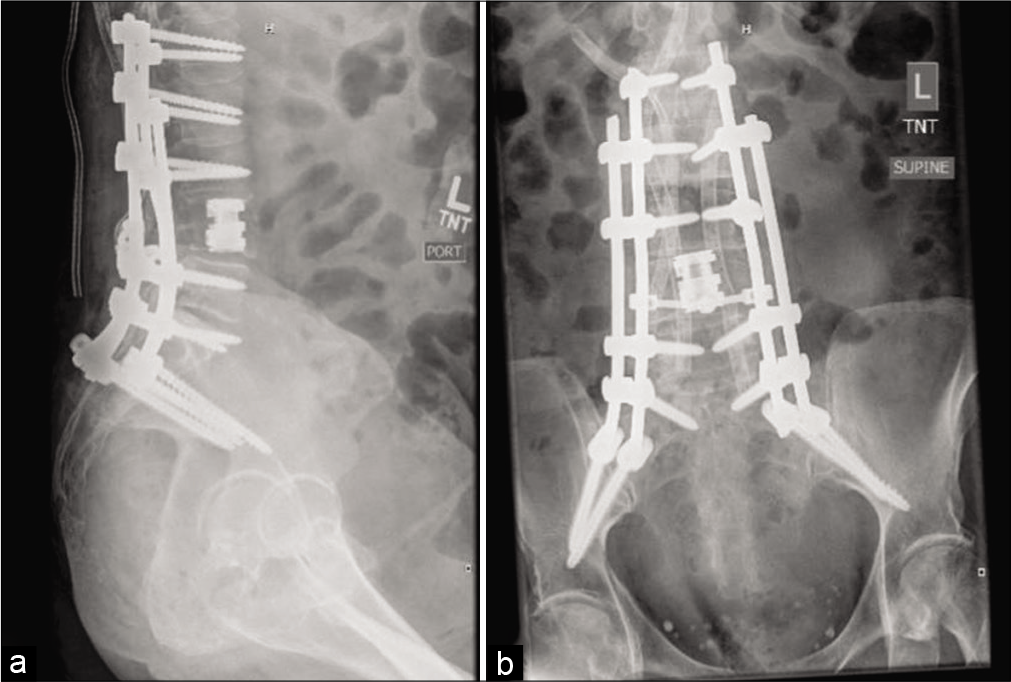
PDAC carries a high mortality rate and rarely metastasizes to the skeletal system. Here, we utilized a posterolateral approach to perform a L3–4 corpectomy and cage placement for resection of the tumor, interbody fusion (L3–4), instrumentation (L1–5), pelvic fixation with bilateral S2-AI instrumentation, and bilateral iliac bolts.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Argentiero A, Calabrese A, Solimando AG, Notaristefano A, Panarelli MM, Brunetti O. Bone metastasis as primary presentation of pancreatic ductal adenocarcinoma: A case report and literature review. Clin Case Rep. 2019. 7: 1972-6
2. Bilsky MH, Laufer I, Fourney DR, Groff M, Schmidt MH, Varga PP. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine. 2010. 13: 324-8
3. Borad MJ, Saadati H, Lakshmipathy A, Campbell E, Hopper P, Jameson G. Skeletal metastases in pancreatic cancer: A retrospective study and review of the literature. Yale J Biol Med. 2009. 82: 1-6
4. Chih YP, Wu WT, Lin CL, Jou HJ, Huang YH, Chen LC. Vertebral compression fracture related to pancreatic cancer with osteoblastic metastasis: A case report and literature review. Medicine (Baltimore). 2016. 95: e2670
5. Iguchi H, Yasuda M, Matsuo T, Sumii T, Funakoshi A. Clinical features and management of pancreatic cancer with bone metastases. Nihon Shokakibyo Gakkai Zasshi. 2004. 101: 872-8
6. Laufer I, Rubin DG, Lis E, Cox BW, Stubblefield MD, Yamada Y. The NOMS framework: Approach to the treatment of spinal metastatic tumors. Oncologist. 2013. 18: 744-51
7. Lin CT, Tang CT, Liu MY, Ma HI. Unusual osteoblastic metastases in the spine secondary to adenocarcinoma of the pancreas. Acta Chir Belg. 2011. 111: 44-5
8. Lunardi S, Muschel RJ, Brunner TB. The stromal compartments in pancreatic cancer: Are there any therapeutic targets?. Cancer Lett. 2014. 343: 147-55
9. Pneumaticos SG, Savidou C, Korres DS, Chatziioannou SN. Pancreatic cancer’s initial presentation: Back pain due to osteoblastic bone metastasis. Eur J Cancer Care (Engl). 2010. 19: 137-40
10. Rades D, Huttenlocher S, Schild SE, Bartscht T. Metastatic spinal cord compression from pancreatic cancer. Anticancer Res. 2014. 34: 3727-30