- Department of Neurosurgery, Mackenzie Evangelical University Hospital, Curitiba, Paraná, Brazil
- CANES Laboratory, University of Miami, Miami, Florida, USA
- Department of Neurosurgery, Santa Casa de Misericórdia de São Paulo, São Paulo, Brazil
- Department of Neurosurgery, Federal University of Paraná, Curitiba, Paraná, Brazil
Correspondence Address:
Luis A. B. Borba, M.D., PhD. Rua Paulo Gorski 1240 #7,CEP 81210220, Department of Neurosurgery, Mackenzie Evangelical University Hospital, Curitiba, Parana, Brazil.
DOI:10.25259/SNI_188_2024
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Guilherme H. W. Ceccato1, Júlia S. de Oliveira1, Pedro H. dos Santos Neto1, Nick. D. Carvalho1, Hugo A. Hasegawa1, Vinícius N. Coelho1, Vitor P. Barreto1, Gustavo A. R. Passos1,2, Paulo A. S. Kadri1, Jean G de Oliveira3, Luis A. B. Borba1,4. Microsurgical resection of a giant exclusively dopamine- secreting jugular foramen paraganglioma: A case report. 25-Apr-2025;16:147
How to cite this URL: Guilherme H. W. Ceccato1, Júlia S. de Oliveira1, Pedro H. dos Santos Neto1, Nick. D. Carvalho1, Hugo A. Hasegawa1, Vinícius N. Coelho1, Vitor P. Barreto1, Gustavo A. R. Passos1,2, Paulo A. S. Kadri1, Jean G de Oliveira3, Luis A. B. Borba1,4. Microsurgical resection of a giant exclusively dopamine- secreting jugular foramen paraganglioma: A case report. 25-Apr-2025;16:147. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13516
Abstract
BackgroundTemporal bone paragangliomas are complex pathologies presenting close relationships with many critical neurovascular structures. Exclusively, dopamine-secreting paragangliomas are rare and may present a major hemodynamic challenge during intraoperative and postoperative periods, with great blood pressure lability due to the dose-dependent properties of dopamine. However, preoperative α or β blockage is usually not advised. Microsurgical resection is the treatment of choice; nevertheless, these tumors commonly present with greater size at diagnosis due to their non-specific clinical manifestations.
Case DescriptionA 43-year-old male patient presented with headache, tinnitus, hearing loss, hypoglossal and facial nerve compromise, as well as vocal cord palsy. Magnetic resonance imaging depicted a giant posterior fossa mass centered in the left jugular foramen extending to the cervical space, associated with important bone erosion. Laboratory investigation depicted elevated serum dopamine concentration of >2.500 pg/mL (reference
ConclusionExclusively dopamine-secreting temporal bone paragangliomas may be successfully resected with a favorable outcome. A multidisciplinary, well-trained team is essential to manage intraoperative challenges up to postoperative rehabilitation adequately. Extensive laboratory training is essential to develop the surgical skills to master this skull-based approach.
Keywords: Case report, Dopamine, Jugular foramen, Paraganglioma, Temporal bone
INTRODUCTION
Head-and-neck paragangliomas (HNPGLs) are neuroendocrine tumors arising from parasympathetic paraganglia, comprising around 0.6% of head and neck neoplasms.[
Exclusively, dopamine-secreting paragangliomas are rare, with <40 cases reported in the literature.[
We present the case of a young male patient with progressive symptoms harboring a giant exclusively dopamine-secreting jugular foramen paraganglioma managed with preoperative embolization followed by microsurgical resection, who presented a favorable outcome.
CASE PRESENTATION
A 43-year-old male presenting progressive headache, hearing loss, and left-sided tinnitus over the past 3 years, with sporadic episodes of left-sided otorrhagia in the last weeks. It was observed on the left side facial nerve palsy House-Brackmann IV, hypoglossal nerve dysfunction, and facial hypoesthesia. Video laryngoscopy depicted left vocal cord palsy [
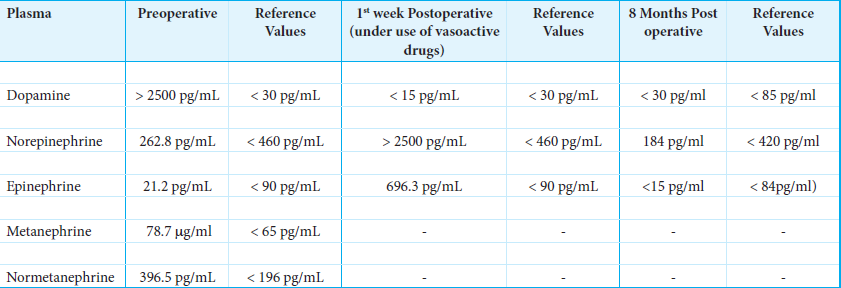
Laboratory investigation demonstrated a high serum concentration of dopamine >2.500 pg/mL (reference <30 pg/mL). Serum values of norepinephrine and epinephrine were in the normal range, 262.8 pg/mL (reference <460 pg/mL) and 21.2 pg/mL (reference <90 pg/mL), respectively [
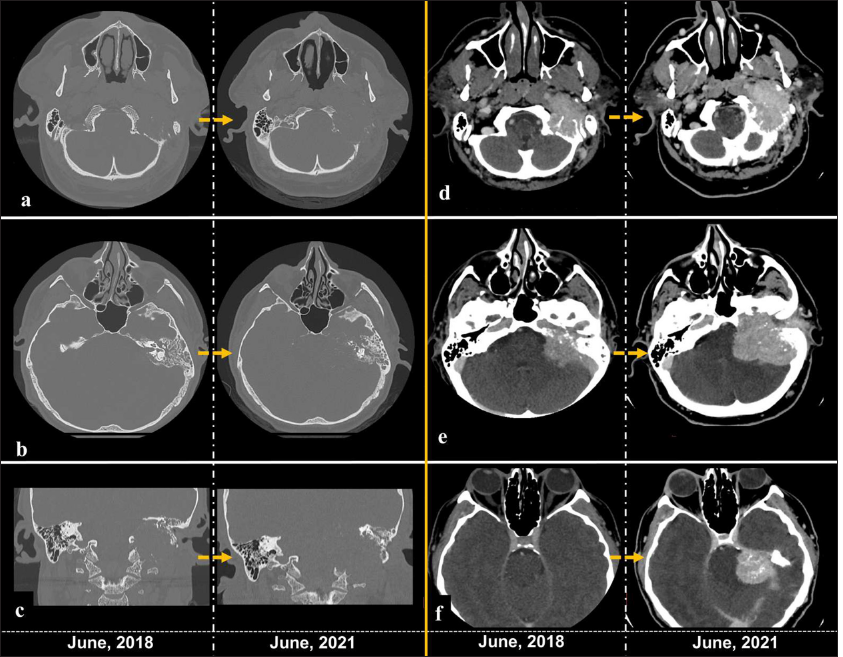
A computed tomography (CT) scan demonstrated a mass centered in the left jugular foramen with extensions toward intra- and extracranial spaces associated with massive bone erosion, including the destruction of middle ear structures. A comparison between CT scans with 3-year intervals; it is demonstrated the growth of the lesion and progression of bone destruction [
Figure 2:
Computed tomography scans comparison between 2018 and 2021. (a-c) Bone window demonstrating the progression of bone erosion over the years, with the greater compromise of the occipital condyle, mastoid, and middle ear structures. (d-f) Postcontrast images depicting the increasing in size of the mass. The yellow arrow means the passage of time between the left and right image.
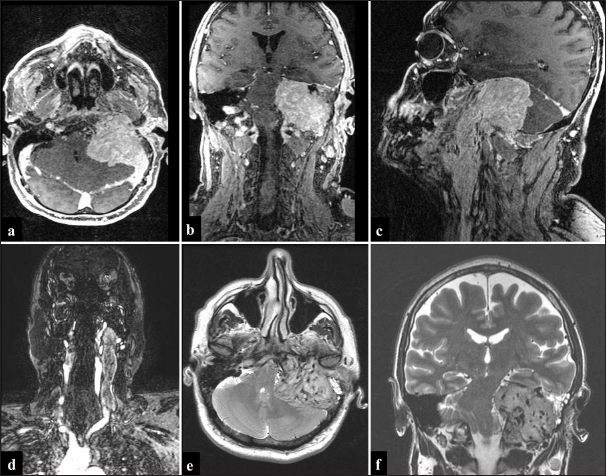
Magnetic resonance imaging (MRI) demonstrated a giant lesion measuring 13 × 4.6 × 8 cm [craniocaudal (CC) × anteroposterior (AP)× latero-lateral (LL)] centered in the left jugular foramen, presenting an important mass effect in the posterior fossa and extracranial extension up to the level of C5–C6 [
Figure 3:
Preoperative magnetic resonance (MR) imaging. (a-c) Post-contrast images expose a large mass with compression of posterior fossa structures and cervical extension through the jugular foramen. (d) MR venography depicts the mass filling the internal jugular vein up to the lower cervical region. (e and f) T2-weighted imaging images depicting many flow voids inside the giant mass, suggestive of a highly vascularized tumor.
The patient underwent angiography and embolization of the mass the day before surgical resection [
Figure 5:
Preoperative embolization. (a-d) Diagnostic angiography demonstrates a highly vascularized mass, with major blood supply from the external carotid (a), ascending pharyngeal (b), and vertebral arteries (c). (d) The angiographic venous phase depicts tumor blush and drainage along the internal jugular vein dilated by the tumor inside it. (e and f) Angio-computed tomography following embolization demonstrates degenerative features inside the mass. The inset image exposes the final embolization view, preserving mainly the superficial temporal artery. (g) 3D model of the tumor, exposing better its vascular relationships and its extensions are also clearly exposed.
Microsurgical resection was performed [
Video 1
Figure 6:
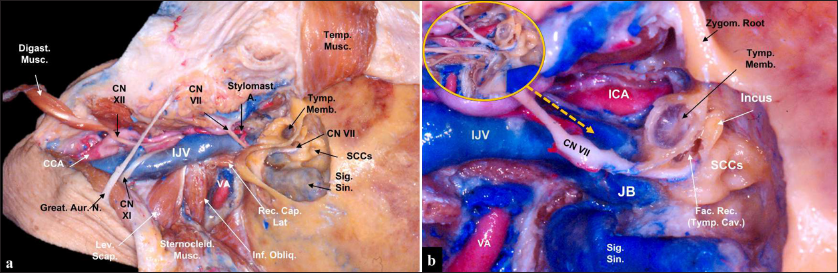
(a) Anatomical view of the surgical approach. It is important to perform a neck dissection to obtain vascular control of major cervical structures. Next, a mastoidectomy is performed, and the extradural tumor may be removed to expose the proximal sigmoid sinus, and the jugular foramen is opened to expose since sigmoid sinus up to the jugular vein. (b) Middle ear structures are accessed through the mastoidectomy, and the incus is an anatomical landmark to point facial canal. A pathway to the middle ear is through the facial recess, anterior to the facial canal, and attention is important as the petrous carotid artery is located deep in this corridor. The great auricular nerve should be harvested from the beginning of dissection to be used to graft the infiltrated facial nerve if necessary. In the inset image (yellow circle)., it is highlighted the lower cranial nerves running underneath the medial wall of the internal jugular vein. CCA: Common carotid artery, CN: Cranial nerve, Digast. Musc.: Digastric muscle, ICA: Internal carotid artery, IJV: Internal jugular vein, Fac. Rec.: Facial recess, Great Aur. N.: Great auricular nerve, Inf. Obliq.: Inferior oblique muscle, JB: Jugular bulb, Lev. Scap.: Levator scapulae muscle, Rec. Cap. Lat.: Rectus capitis lateralis muscle, SCCs: Semicircular canals, Sig. Sin.: Sigmoid sinus, Sternocleid. Musc.: Sternocleidomastoid muscle, Stylomast. A.: Stylomastoid artery, Tymp. Cav.: Tympanic cavity, Tymp. Memb.: Tympanic membrane, VA: Vertebral artery, Zygom. Root: Zygomatic root.
The patient is positioned supine with the head rotated to the contralateral side and extended to increase the sub/retromandibular space. An arciform incision is performed starting from the level of the pinna to the medial border of the sternocleidomastoid muscle. Following the reflection of the skin flap, the great auricular nerve is identified and dissected along its trajectory over the sternocleidomastoid muscle to be used if necessary for facial nerve reconstruction. Temporalis fascia is dissected from the muscle and reflected, kept attached to the sternocleidomastoid muscle, which is mobilized posteriorly. The next step is to obtain neurovascular control in the neck, and the common, internal and external carotid arteries are identified, as well as the internal jugular vein. The external carotid artery branches involved with the tumor blood supply can be ligated. In the cervical area, it is possible to identify the hypoglossal nerve crossing over carotid bifurcation and giving its branch to the ansa cervicalis; accessory nerve runs posteriorly over the internal jugular vein; vagus nerve travels within the carotid sheath between internal carotid artery and internal jugular vein; and glossopharyngeal nerve runs anteriorly crossing over the lateral aspect of distal cervical segment of internal carotid artery.
After cervical dissection, surgery moves to the mastoidectomy. Initially, the antrum is opened for helping to identify the lateral semicircular canal, and then the other semicircular canals; the sigmoid sinus is also skeletonized. Incus is identified in front of semicircular canals and points to the facial canal. In this case, as the patient presented preoperative facial nerve palsy, the canal will be opened to expose and decompress the facial nerve, which will be later grafted with the great auricular nerve if necessary. Dissection includes the removal of the mastoid tip to allow exposure of the whole trajectory of the facial nerve from the mastoid to the extracranial segment. Furthermore, the sigmoid sinus is all the way skeletonized up to the internal jugular vein, including the jugular bulb. All venous outflow is exposed, and the rectus capitis lateralis muscle is mobilized as it relates to the posterior border of the jugular foramen, and if necessary, anterolateral dissection reaches the styloid process. An extradural tumor is first resected, including tumors involving the facial nerve. Extension to the middle ear can be reached through the facial recess, between the facial nerve and chorda tympani. The tumor infiltrates and projects into the lumen of the sigmoid sinus up to the internal jugular vein, and first sigmoid sinus is incised to remove the proximal intraluminal tumor, and then, it is packed and ligated. As the patient already presented several lower cranial nerve deficits, the internal jugular vein is ligated distal to tumor projection, and the whole vein is gradually resected. However, if the patient had preserved lower cranial nerve function, the medial wall of the vein would be preserved. An intrabulbar resection may be performed in the jugular bulb, and bleeding from some emissary veins, especially from the inferior petrosal sinus, may be present and it is controlled with fibrin glue and hemostatic material. The intradural tumor is finally removed, with care to preserve surrounding arachnoid planes.
The closure should be meticulous, employing vascularized flaps as the previous pericranium and temporalis fascia harvested, and the cavity is filled with fat, and fibrin glue. Mastoid air cells should be packed with muscle pieces. Layer by layer must be approximated to avoid most cerebrospinal fluid leaks. A preemptive lumbar drain may be considered, if necessary, in the immediate postoperative period.
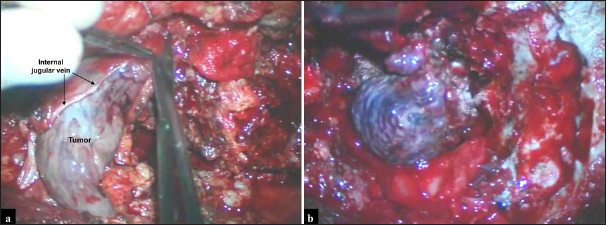
From the middle to the end of the procedure, the patient presented severe blood pressure lability, varying from deep to high values without apparent cause, and demanding usage of an infusion pump of vasoactive drugs alternating norepinephrine, nitroprusside, esmolol, and dopamine recurrently. We removed the intradural and extradural tumor projections, including extension inside the lumen of internal jugular vein and sigmoid sinus, after ligation of these structures [
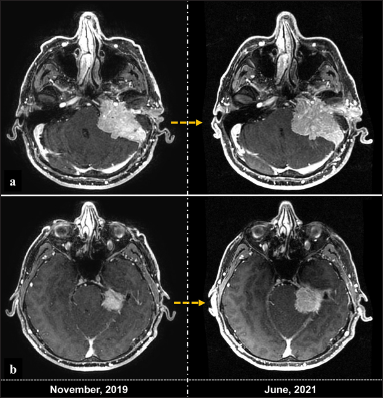
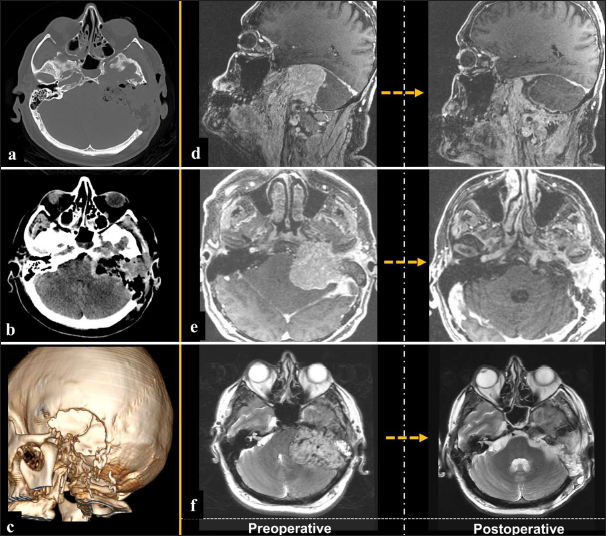
Postoperative imaging demonstrated complete tumor resection [
DISCUSSION
Jugular foramen paragangliomas are complex pathologies that can involve both intra- and extracranial spaces, as well as intra- and extradural compartments, and can infiltrate inside of the internal jugular vein and sigmoid sinus, beyond surrounding critical neurovascular structures. Around 40% of HNPGLs are associated with familial disease and the most common mutation is related to a subunit (A-D) of the enzyme succinate dehydrogenase involved in the Krebs cycle, and a germline mutation is commonly identified.[
Especially in patients with apparent adrenergic symptoms is important to screen for other possible synchronic tumors, as HNPGLs are mostly associated with the parasympathetic system, while thoracic, abdominal, and pelvic tumors are more likely linked with the sympathetic system.
Exclusively, dopamine-secreting tumors are rare, and these tumors have a median age of 48 years at diagnosis and a male-to-female ratio of 1:1.5.[
Dopamine has a dose-dependent hemodynamic effect, varying from a vasodilatory effect at low concentrations up to inotropic effects at moderate dosage through β-receptor stimulation and, at higher concentrations, a vasopressor effect due to α receptor affinity.[
Management of HNPGLs includes wait-and-scan, radiosurgery, and microsurgical resection; however, the therapeutic decision should be individualized in each case.[
CONCLUSION
Exclusively dopamine-secreting paragangliomas may be successfully resected; however, it is essential to be well-prepared to deal with possible major hemodynamic instability. It can occur since preoperative embolization, especially during the intraoperative period, and be apparent up to the 1st days following microsurgical resection. Laboratory training is essential to the surgeon’s better understanding of surgical approaches to jugular foramen, and a multidisciplinary team is critical to manage these patients adequately.
Author contributions
Gustavo A. R. Passos: Conceptualization, Data collection, Data analysis, Final article review. Paulo A. S. Kadri: Conceptualization, Data collection, Data analysis, Final article review. Jean G. de Oliveira: Conceptualization, Data collection, Data analysis, Final article review. Dr Gustavo, Dr Paulo and Dr Jean contributed to review the video article and the clinical case, highlight surgical nuances and correlate with literature findings.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Video available on
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Castelhano L, Correia1 F, Donato S, Ferreira L, Montalvão P, Magalhães M. Head and neck paragangliomas: The experience of a southern European cancer center. Acta Méd Port. 2022. 35: 789-97
2. Ceccato GH, Rassi MS, Borba LA. Microsurgical resection of multiple giant glomus tumors. J Neurol Surg B Skull Base. 2019. 80: S385-8
3. Ceccato GH, Foltran RS, Franke K, Dias M, Sallé M, Borba LA. Transmastoid/infralabyrinthine approach to the jugular foramen: 2-dimensional operative video. Oper Neurosurg. 2023. 25: e361-2
4. Cleere EF, Martin-Grace J, Gendre A, Sherlock M, O’Neill JP. Contemporary management of paragangliomas of the head and neck. Laryngoscope Investig Otolaryngol. 2022. 7: 93-107
5. Erickson D, Kudva YC, Ebersold MJ, Thompson GB, Grant CS, Van Heerden JA. Benign paragangliomas: Clinical presentation and treatment outcomes in 236 patients. J Clin Endocrinol Metab. 2001. 86: 5210-6
6. Foo SH, Chan SP, Ananda V, Rajasingam V. Dopamine-secreting phaeochromocytomas and paragangliomas: Clinical features and management. Singapore Med J. 2010. 51: e89-93
7. Hayashi T, Ozgur M. Head and neck paragangliomas: What does the pathologist need to know?. Diagn Histopathol. 2014. 20: 316-25
8. Jansen TT, Timmers HJ, Marres HA, Kunst HP. Feasibility of a wait-and-scan period as initial management strategy for head and neck paraganglioma. Head Neck. 2017. 39: 2088-94
9. Lin EP, Chin BB, Fishbein L, Moritani T, Montoya SP, Ellika S. Head and neck paragangliomas: An update on the molecular classification, state-of-the-art imaging, and management recommendations. Radiol Imaging Cancer. 2022. 4: e210088
10. Mete O, Wenig BM, editors. Update from the 5th edition of the world health organization classification of head and neck tumors: Overview of the 2022 WHO classification of head and neck neuroendocrine neoplasms. Head Neck Pathol. 2022. 16: 123-42
11. Michałowska I, Ćwikła JB, Michalski W, Wyrwicz LS, Prejbisz A, Szperl M. Growth rate of paragangliomas related to germline mutations of the SDHX genes. Endocr Pract. 2017. 23: 342-52
12. Miyamoto S, Yoshida Y, Ozeki Y, Okamoto M, Gotoh K, Masaki T. Dopamine-secreting pheochromocytoma and paraganglioma. J Endocr Soc. 2021. 5: bvab163
13. Pellitteri PK, Rinaldo A, Myssiorek D, Gary Jackson C, Bradley PJ, Devaney KO. Paragangliomas of the head and neck. Oral Oncol. 2004. 40: 563-75
14. Prasad SC, Paties CT, Schiavi F, Esposito DL, Lotti LV, MarianiCostantini R, MarianiCostantini R, editors. Tympanojugular paragangliomas: Surgical management and clinicopathological features. Paraganglioma: A multidisciplinary approach. Brisbane, Australia: Codon Publications; 2019. p. 99-123
15. Sandow L, Thawani R, Kim MS, Heinrich MC. Paraganglioma of the head and neck: A review. Endocr Pract. 2023. 29: 141-7
16. Taïeb D, Wanna GB, Ahmad M, Lussey-Lepoutre C, Perrier ND, Nölting S. Clinical consensus guideline on the management of phaeochromocytoma and paraganglioma in patients harbouring germline SDHD pathogenic variants. Lancet Diabetes Endocrinol. 2023. 11: 345-61
17. Williams MD. Paragangliomas of the head and neck: An overview from diagnosis to genetics. Head Neck Pathol. 2017. 11: 278-87
18. Wu S, Chen W, Shen L, Xu L, Zhu A, Huang Y. Risk factors for prolonged hypotension in patients with pheochromocytoma undergoing laparoscopic adrenalectomy: A single-center retrospective study. Sci Rep. 2017. 7: 5897
19. Yi JW, Oh EM, Lee KE, Choi JY, Koo Do H, Kim KJ. An exclusively dopamine secreting paraganglioma in the retroperitoneum: A first clinical case in Korea. J Korean Surg Soc. 2012. 82: 389-93