- Department of Endovascular Neurosurgery, Neurosurgery and Spine Surgery, 1st of October Regional Hospital, ISSSTE, Mexico City, Mexico
- Department of Endovascular Neurosurgery, Stroke Team Mexico, Mexico City, Mexico
- Department of Pediatric Neurosurgery, Neurosurgery and Spine Surgery, 1st of October Regional Hospital, ISSSTE, Mexico City, Mexico
- Stroke Team Mexico, American Association of Neurological Surgeons Student Chapter, Autonomous University of the State of Hidalgo, Hidalgo, Mexico
Correspondence Address:
José de Jesús Gutiérrez-Baños, Department of Endovascular Neurosurgery, ISSSTE Hospital Regional 1ro de Octubre, Av Instituto Politécnico Nacional 1669, México City, Mexico.
DOI:10.25259/SNI_979_2024
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: José de Jesús Gutiérrez-Baños1,2, Carlos Castillo-Rangel1, Mauricio Ivan Rodriguez-Pereira2,3, Jaime Ordoñez Granja1, Daniel Oswaldo Dávila-Rodríguez1, Jecsán Tovar-Fuentes4, Alondra Sarai Tovar-Jiménez4, Juan Alberto Hernández-López4. Modified orbitofrontal approach for optic nerve sheath hemangioma: Illustrative case and literature review. 07-Feb-2025;16:35
How to cite this URL: José de Jesús Gutiérrez-Baños1,2, Carlos Castillo-Rangel1, Mauricio Ivan Rodriguez-Pereira2,3, Jaime Ordoñez Granja1, Daniel Oswaldo Dávila-Rodríguez1, Jecsán Tovar-Fuentes4, Alondra Sarai Tovar-Jiménez4, Juan Alberto Hernández-López4. Modified orbitofrontal approach for optic nerve sheath hemangioma: Illustrative case and literature review. 07-Feb-2025;16:35. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13376
Abstract
Background: Optic nerve sheath hemangiomas (ONSHs) are rare vascular tumors from the blood vessels surrounding the optic nerve, accounting for
Case Description: We present the case of a 57-year-old woman who experienced progressive left-eye swelling, increased tearing, and declining visual acuity. Imaging studies, including MRI and computed tomography scans, revealed a left intraconal mass suggestive of ONSH. A transcranial-modified orbitofrontal approach was employed for tumor resection due to its proximity to vital structures. Postoperative histopathology confirmed hemangioma. At 1-year follow-up, the patient exhibited significant improvement in visual function and resolution of orbital swelling.
Conclusion: This case highlights the critical role of surgical intervention in managing ONSHs that threaten visual function and cause mass effects. The transcranial-modified orbitofrontal approach proved effective in providing optimal access for safe tumor resection and improving visual outcomes. Integrating advanced imaging techniques and intraoperative monitoring contributes significantly to enhancing prognosis in ONSH cases.
Keywords: Fronto-orbital approach, Optic nerve sheath hemangioma, Orbital approach, Surgical decompression, Visual impairment
INTRODUCTION
Epidemiology and etiology
Optic nerve sheath hemangiomas (ONSHs) are rare benign vascular tumors that develop within the sheath of the optic nerve. These tumors are typically slow-growing, with symptoms often not manifesting until the tumor reaches considerable size. The pathophysiology of ONSHs involves endothelial cell proliferation, frequently influenced by factors like vascular endothelial growth factor (VEGF), although the precise molecular mechanisms remain an area of active research.[
Clinical manifestations
Clinically, ONSHs present with symptoms such as progressive vision loss, optic disc swelling, and proptosis, although some cases may remain asymptomatic for an extended period. Early diagnosis through imaging, particularly magnetic resonance imaging (MRI) with gadolinium enhancement, is essential to evaluate the size, location, and characteristics of the tumor.[
Diagnostic methods
MRI is considered the gold standard for diagnosing ONSHs, providing detailed anatomical views, and detecting tumor extension.[
Given the rarity of ONSHs and their potential for significant visual impairment, surgical intervention is often necessary. Treatment options vary depending on tumor location and size, ranging from observation to more invasive approaches such as endonasal resection, radiation therapy, or craniotomy.[
In this report, we describe the case of a 57-year-old female with ONSH who underwent successful tumor resection using a transcranial modified orbitofrontal approach. This technique involves a single-piece craniotomy with lateral and superior orbital osteotomy, providing optimal exposure to the tumor while minimizing risks associated with brain retraction and damage to surrounding tissues, which is consistent with findings from other studies on similar orbital tumors.[
Histopathological features
Cavernous intraocular hemangiomas are characterized by large vascular spaces lined with mature endothelium and a dense stromal component. Acute thrombosis is a common feature, with fibrin clots encased in CD31-positive endothelium. Perivascular areas show increased cellularity with CD31+ and VEGFr2+ staining, while regions of Ki67 positivity indicate active proliferation. The presence of CD68+ cells signals inflammatory activity. In addition, the stroma contains CD31+ microcapillaries and smooth muscle cells marked by smooth muscle actin (SMA) staining, which integrate into the vascular architecture.[
Molecular pathogenesis
VEGF plays a crucial role in the progression of hemangiomas. In these tumors, VEGF expression is elevated and contributes to neovascularization and tumor growth. VEGF exists in several isoforms through alternative splicing, with VEGF-165 being the predominant form in hemangiomas, VEGF-121 and VEGF-165 being secreted, while VEGF-189 and VEGF-206 are matrix-bound.[
Hypoxia, a common feature in tumors, triggers the stabilization of hypoxia-inducible factor 1α (HIF-1α), which activates VEGF expression.[
VEGF functions as a mitogen for endothelial cells and drives neovascularization, but the resulting blood vessels in hemangiomas are often structurally abnormal and highly permeable, fostering tumor growth and enhancing tumor expansion. Secondarily, altered tumor vasculature promotes immune evasion by altering tumor vasculature and impairing immune cell infiltration, creating an immunosuppressive microenvironment that allows the tumor to escape immune surveillance.[
Treatment options
Surgical Management: Surgery remains the primary treatment modality for ONSHs, particularly in symptomatic cases or those with progressive visual loss. The choice of surgical approach depends on tumor size, location within the optic nerve sheath, and the degree of intraorbital extension. Key surgical techniques include:
Transcranial approaches
Fronto-orbito-zygomatic (FOZ) approach
This approach provides excellent exposure to the optic nerve sheath and orbital apex, involving a combined frontal, orbital, and zygomatic osteotomy. It allows for decompression of the optic canal and excision or debulking of the tumor while minimizing brain manipulation.[
Modified orbito-frontal approach
This variation involves similar principles but preserves the zygomatic arch, offering a less invasive option while providing adequate access for tumor resection or biopsy.[
Endoscopic approaches
Transethmoidal-transsphenoidal approach
Although less commonly used, this approach may be suitable for select cases of anterior skull base extension. It allows access to the optic canal and intracranial portion of the optic nerve sheath with minimal morbidity.[
Intraoperative neurophysiological monitoring
To minimize postoperative neurological deficits, intraoperative neurophysiological monitoring is essential during surgery near the optic nerve and chiasm, ensuring real-time assessment of visual evoked potentials and cranial nerve function.[
CASE PRESENTATION
Patient history and clinical findings
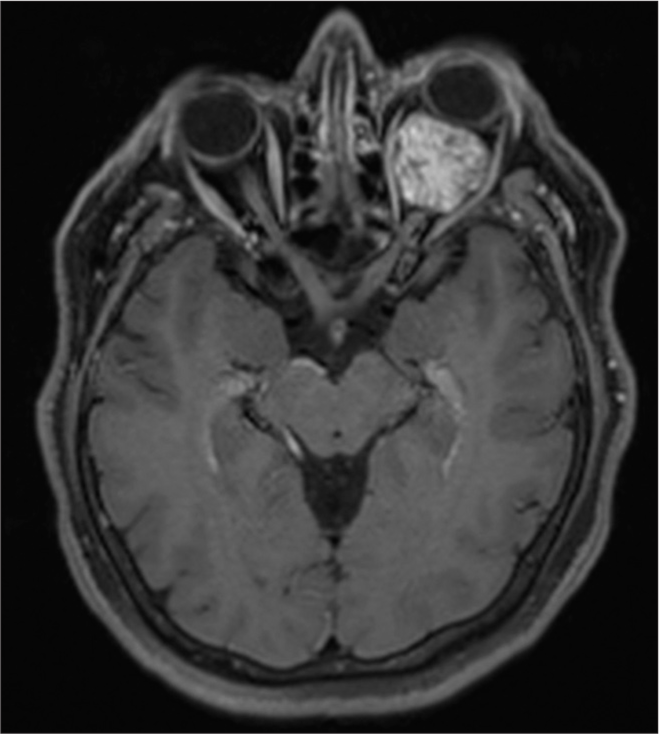
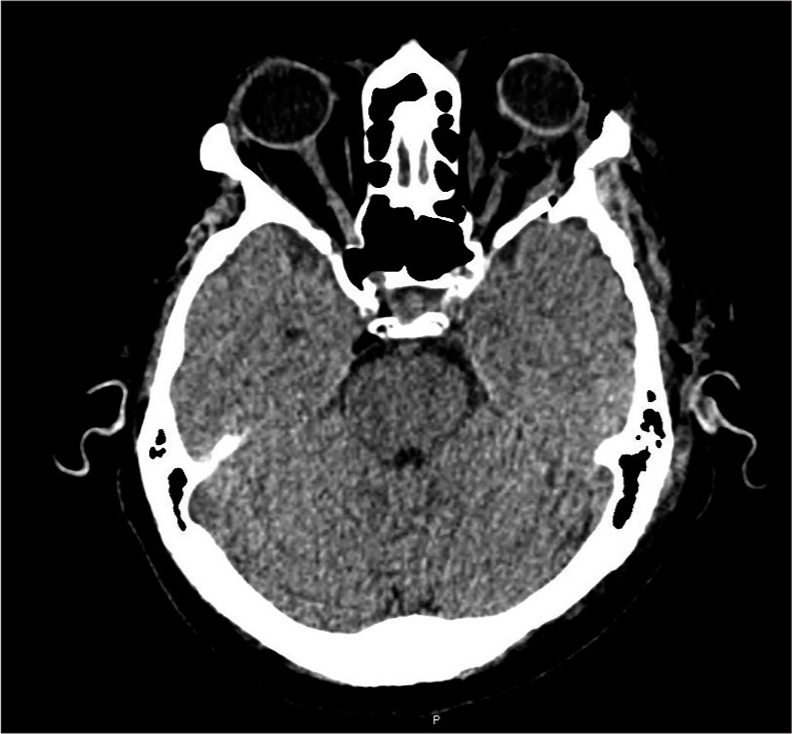
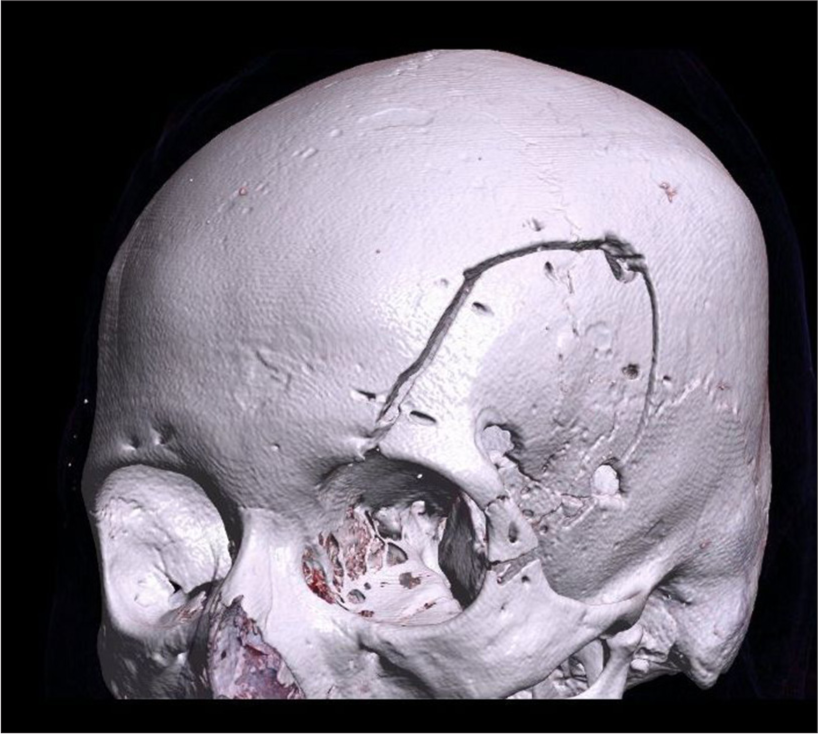
A 57-year-old female presented with a 2-year history of progressive left eye swelling, epiphora, conjunctival injection, and gradual visual acuity decline to perception of light. The ophthalmological examination revealed optic disc edema and decreased visual acuity in the left eye. Pre-surgical visual acuity was measured using the Snellen test, with a result of 20/25 in the right eye and 20/30 in the left eye, both outside the normal range. Imaging studies, including MRI and computed tomography, confirmed a left intraconal mass exerting mass effect on the optic nerve and globe, consistent with optic nerve sheath meningioma [
Imaging studies
Gd-MRI demonstrated a well-circumscribed intraconal lesion along the left optic nerve sheath.
Surgical approach
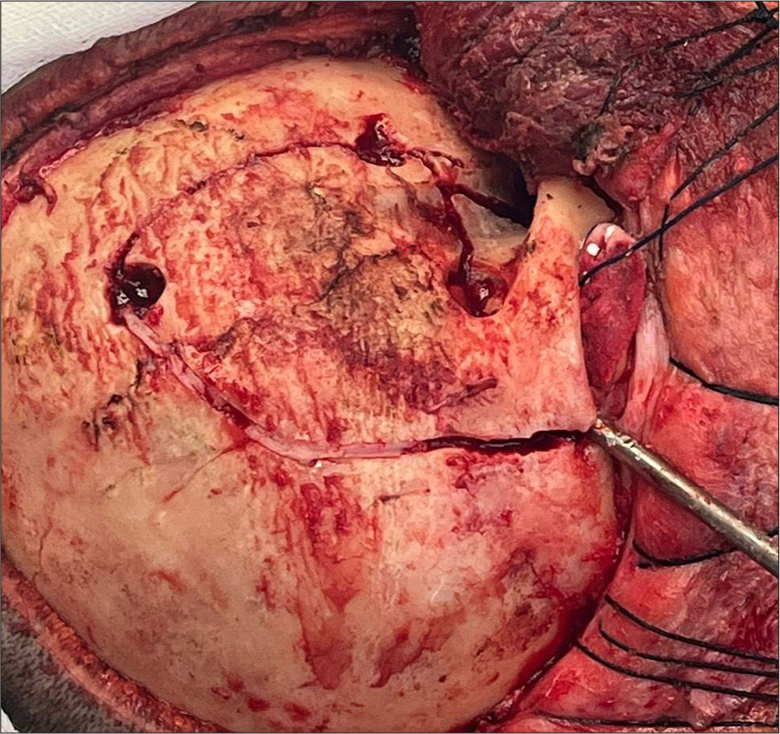
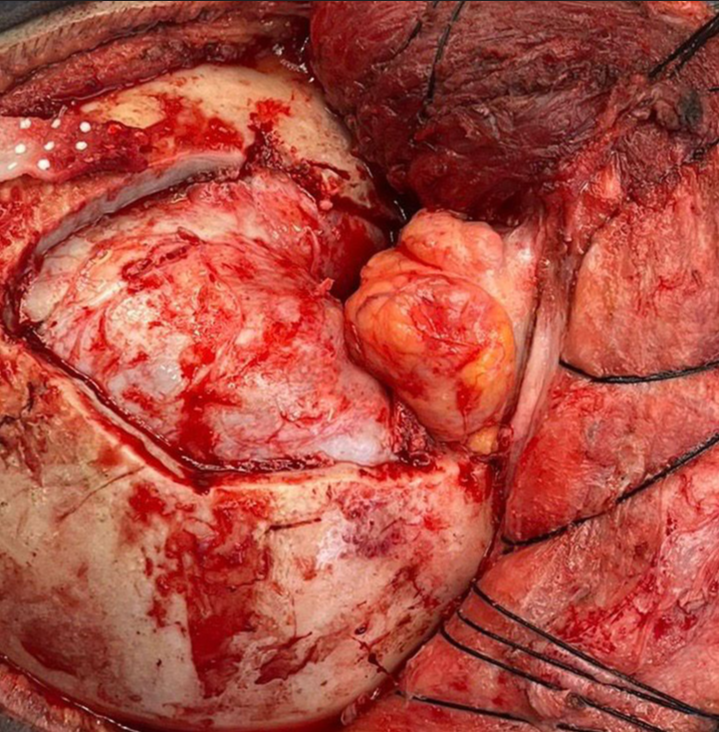
Given the tumor’s location and extent within the left orbit, a transcranial-modified orbitofrontal approach was chosen. This technique involved a single-piece craniotomy with lateral and superior orbital osteotomy, providing adequate exposure to the optic canal and intraconal space while minimizing brain retraction. Intraoperatively, careful dissection was performed to separate the tumor from the adjacent optic nerve and extraocular muscles, allowing for maximal safe resection [
Histopathological examination
The postoperative histopathological examination confirmed the diagnosis of a cavernous nodular fibrohemangioma located in the left intraorbital region, consistent with ONSH. Macroscopically, the specimen presented as a dense, ovoid tissue structure measuring 2 × 1.8 cm with a grayish, uniformly firm, rubbery surface. Serial sections revealed a congestive, fibrous tissue thickness with areas suggestive of necrosis. Microscopically, findings included subacute intraluminal inflammatory changes along with scattered dystrophic and degenerative calcifications.
Postoperative course and follow-up
The patient’s postoperative course was uneventful, with a resolution of orbital swelling and improvement in visual acuity. While the updated or new ophthalmological scales for assessing postoperative visual outcomes were not included in the ophthalmology report, the patient’s progress was closely monitored. Regular ophthalmological assessments and imaging studies were conducted to track tumor recurrence or residual disease [
DISCUSSION
ONSHs are rare, and their molecular pathogenesis remains an area of active research. It is believed that mutations in genes regulating endothelial cell proliferation, such as VEGF, contribute to the formation of these vascular tumors.[
The modified orbitofrontal approach has emerged as a promising technique for managing ONSHs, especially in cases where there is progressive visual deterioration and mass effect in the intraorbital region. This approach’s ability to decompress the orbital roof and lateral wall while providing clear access to intraorbital lesions is in line with findings from other studies that highlight its effectiveness in treating orbital tumors, including hemangiomas.[
Optimal treatment strategies
The management of ONSHs requires individualized treatment strategies that take into account tumor size, location, and patient-specific factors such as age and comorbidities. As demonstrated in other reports, surgical resection remains the cornerstone of treatment, with the goal of complete tumor removal while preserving visual function.[
Alternative treatment strategies
Observation can be used in patients with stable or negligible visual decline and minimal tumor progression. This approach avoids surgical risks, but the ONSH growth may affect visual improvement after treatment and the potential for progressive vision loss. Moreover, this treatment needs control imaging evaluation (MRI) and visual function assessment for years to intervene if progression occurs.[
Radiotherapy, especially stereotactic radiosurgery (SRS), is an effective option when patients have unresectable tumors, residual disease, or recurrence. This approach provides excellent local tumor control and minimal collateral damage.[
Endoscopic approaches, like the transethmoidal transsphenoidal route, offer a minimally invasive option for cases with anterior skull base extension. These techniques minimize morbidity and avoid extensive craniotomy, but they are limited to specific tumor locations, restricting their widespread use.[
This study highlights the advantages of the Modified Orbito-Frontal Approach, which offers enhanced access to the optic nerve sheath and orbital apex while minimizing brain manipulation. Compared to the FOZ Approach, this technique reduces surgical time and postoperative complications, with improved preservation of visual function. Endoscopic techniques and radiotherapy are less invasive alternatives; however, they lack the decompressive benefits of surgical resection, restricting their utility to select clinical contexts.[
ROLE OF ADJUVANT THERAPY
In cases where complete resection is not feasible or in the presence of residual or recurrent tumors, adjuvant therapies may be considered, including SRS and conventional radiotherapy. Studies have shown that SRS can be particularly effective in treating small residual lesions or recurrent tumors, providing a targeted approach while minimizing damage to surrounding healthy tissue.[
Challenges in management and limitations
Despite significant advances in diagnostic imaging and surgical techniques, managing ONSHs remains challenging, particularly when tumors are located near critical neurovascular structures. As highlighted by recent studies, the proximity of ONSHs to the optic nerve, carotid artery, and other essential structures requires meticulous planning to minimize surgical complications.[
Future directions and research
Future research on ONSHs should focus on elucidating the molecular markers and genetic pathways associated with their pathogenesis. This would provide critical insights into the development of targeted therapies and personalized treatment approaches, as suggested by recent studies on other vascular anomalies.[
CONCLUSION
ONSHs are rare, challenging tumors that require prompt diagnosis and tailored treatment. The modified orbitofrontal approach is particularly beneficial for patients experiencing painless visual deterioration and intraorbital mass effect for several reasons. First, it is less invasive compared to other surgical techniques while still offering excellent visibility of the orbital contents. This enables targeted removal of bone and tumor tissue, which helps preserve the patient’s visual function and reduces postoperative morbidity. In addition, by removing bone fragments from the orbital roof and lateral wall, this approach effectively decompresses the optic nerve and surrounding vital structures, which is crucial for alleviating the mass effect caused by the tumor, potentially improving or stabilizing visual acuity. Finally, the direct access provided by the orbitofrontal approach allows for a more precise and complete resection of the tumor, which is particularly important in cases where the lesion is situated in challenging locations within the orbit. This clear operative field is essential for avoiding damage to critical structures, making the modified orbitofrontal approach a preferred option in these clinical scenarios.
Ethical approval
The Institutional Review Board has waived the ethical approval for this study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: Beyond discovery and development. Cell. 2019. 176: 1248-64
2. Cennamo G, Comune C, Cennamo G, de Crecchio G. Multimodal imaging of optic nerve head capillary hemangioma. Retina. 2018. 38: e50-2
3. DeHart A, Richter G. Hemangioma: Recent advances. F1000Res. 2019. 8: F1000 Faculty Rev-1926
4. Eckstein A, Welkoborsky HJ. Interdisziplinäre therapie der erkrankungen der orbita [Interdisciplinary management of orbital diseases]. Laryngorhinootologie. 2024. 103: S43-99
5. ISSVA classification of vascular tumors and vascular malformations. International Society for the Study of Vascular Anomalies. Available from: https://www.issva.org/userfiles/file/issva-classification-2018.pdf [Last accessed on 2024 Nov 14].
6. Kane G, Fernandez-Pineda I. Targeted therapies for vascular malformations. Front Med. 2024. 11: 1446046
7. Kaspi M, Gain P, Thuret G, Garcin T. A holy ball for the optic nerve head: Aesthetic capillary hemangioma. J Franç Ophtalmol. 2021. 44: 1460-3
8. Kunimoto K, Yamamoto Y, Jinnin M. ISSVA classification of vascular anomalies and molecular biology. Int J Mol Sci. 2022. 23: 2358
9. Machein MR, Plate KH. VEGF in brain tumors. J NeuroOncol. 2000. 50: 109-20
10. Mabeta P, Steenkamp V. The VEGF/VEGFR axis revisited: Implications for cancer therapy. Int J Mol Sci. 2022. 23: 15585
11. Maus M, Goldman HW. Removal of orbital apex hemangioma using new transorbital craniotomy through suprabrow approach. Ophthalmic Plast Reconstr Surg. 1999. 15: 166-70
12. Milman T, Grossniklaus HE, Goldman-Levy G, Kivelä TT, Coupland SE, White VA, editors. The 5th edition of the World Health Organization classification of tumours of the eye and orbit. Ocular Oncol Pathol. 2023. 9: 71-95
13. Poloschek CM, Lagrèze WA, Ridder GJ, Hader C. Klinische und neuroradiologische diagnostik bei orbitatumoren [Clinical and neuroradiological diagnostics of orbital tumors]. Ophthalmol. 2011. 108: 510-8
14. Reyes-Soto G, Carrillo-Hernández JF, Cacho-Díaz B. Surgical treatment of orbital tumors in a single center: Analysis and results. Surg Neurol Int. 2024. 15: 122
15. Statler B, Kosmorsky G, Rachitskaya A. Swept source OCT angiography of optic nerve head retinal capillary hemangioma. Ophthalmol Retina. 2020. 4: 822
16. Stoyukhina AS, Andzhelova DV, Yusef Y. Gemangiomy diska zritel’nogo nerva [Hemangiomas of the optic nerve head]. Vest Oftalmol. 2022. 138: 66-78
17. Torrence D, Antonescu CR. The genetics of vascular tumours: An update. Histopathology. 2022. 80: 19-32
18. Xiang S, Gong X, Qiu T, Zhou J, Yang K, Lan Y. Insights into the mechanisms of angiogenesis in infantile hemangioma. Biomed Pharmacother. 2024. 178: 117181