- Department of Neurosurgery, Brigham and Women’s Hospital, Boston, United States
- Department of Otolaryngology Head and Neck Surgery, Brigham and Women’s Hospital, Boston, United States
- Department of Neurology, Massachusetts General Hospital, Boston, United States
Correspondence Address:
Christopher S Hong, Department of Neurosurgery, Brigham and Women’s Hospital, Boston, United States, 60 Fenwood Road, BTM 4, Boston, MA 02115.
DOI:10.25259/SNI_719_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Christopher S Hong1, Jakob V E Gerstl1, C Eduardo Corrales2, Timothy R Smith1, Eva K Ritzl3. Monitoring of visual-evoked potentials during fat packing in endoscopic resection of a giant pituitary adenoma. 25-Oct-2024;15:387
How to cite this URL: Christopher S Hong1, Jakob V E Gerstl1, C Eduardo Corrales2, Timothy R Smith1, Eva K Ritzl3. Monitoring of visual-evoked potentials during fat packing in endoscopic resection of a giant pituitary adenoma. 25-Oct-2024;15:387. Available from: https://surgicalneurologyint.com/surgicalint-articles/monitoring-of-visual-evoked-potentials-during-fat-packing-in-endoscopic-resection-of-a-giant-pituitary-adenoma/
Abstract
Background:Endoscopic transsphenoidal surgery has become a mainstay surgical approach for sellar pathologies and can effectively decompress mass effects on the optic nerves. Visual-evoked potentials (VEPs) have been utilized as an intraoperative adjunct during endoscopic transsphenoidal surgery to monitor the integrity of the optic pathways, but the data surrounding its reliability and efficacy remain heterogeneous.
Case Description:An 80-year-old male underwent endoscopic transsphenoidal resection of a pituitary macroadenoma with preoperative visual deficits related to optic nerve compression. During fat packing of the resection cavity, a decrease in VEPs was noted, which seemingly improved after partial fat removal, although with paradoxically reduced VEP latencies. Despite this, the patient developed a visual field deficit postoperatively, requiring re-operation for further removal of the fat packing.
Conclusion:This was a case of initially poorly formed VEPs that deteriorated and apparently improved following surgical intervention. The finding of shortened latencies of the VEPs was likely from noise contamination, creating the illusion of improved signal amplitudes. We recommend careful assessment of VEP data for baseline reproducibility, particularly in patients with pre-existing visual field deficits. Appropriate anesthetic selection is also important to reduce noise interference from the electroencephalogram.
Keywords: Endoscopic, Neuromonitoring, Pituitary adenoma, Visual-evoked potentials
INTRODUCTION
Endonasal transsphenoidal surgery remains the gold standard for pituitary tumor surgery. A key consideration during this surgical approach is the proximity of the normal pituitary gland and nearby pathologies to the optic nerves, and as such, ample care must be taken to avoid iatrogenic damage to the optic apparatus.
Visual-evoked potentials (VEPs) have been a longstanding method of monitoring the electrophysiologic activity of the visual pathways from the optic nerves to the occipital cortex for a variety of neurological pathologies. Historically, VEPs have been used widely in neuroophthalmology to diagnose and monitor various disease states such as multiple sclerosis, ischemic optic neuropathy, and various neurodegenerative diseases, among others.[
Briefly, to record VEP waveforms, visual stimuli are presented to the right and left eye using goggles. Flashes can be used while the eyelids are closed during surgery. Adequate stimulation is assured by electroretinogram (ERG) recordings from electrodes placed around the eye. The VEPs are subsequently recorded from needle electrodes placed on the occipital scalp. For intraoperative monitoring, each patient serves as his or her control. Signals are elicited and recorded during the entire surgery and compared to baseline data. Changes in wave morphology, such as decreased amplitude or increased latency, indicate potential pathological insults to the optic pathways.
VEPs can be difficult to interpret during transsphenoidal surgeries. The OR environment can introduce noise. Criteria for the interpretation of VEPs during surgery have been suggested but are not uniform.[
At our institution, we routinely utilize intraoperative VEPs for all patients undergoing endoscopic transsphenoidal surgery. While our experience has been overall very reliable in the correlation of VEPs with clinical outcomes, in this report, we describe a case of overpacking of the sellar resection cavity with fat that led to a postoperative visual deficit despite the seemingly reassuring reversal of the intraoperatively observed changes in the VEPs. We discuss the possible reasons why the VEPs were less reliable in this case and suggest strategies to avoid repeating this scenario.
CASE DESCRIPTION
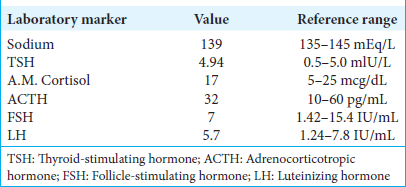
An 80-year-old male was referred to our institution after a large pituitary lesion was seen on magnetic resonance imaging (MRI), obtained as a part of a work-up for short-term memory loss. A full panel of endocrine labs was within normal limits [
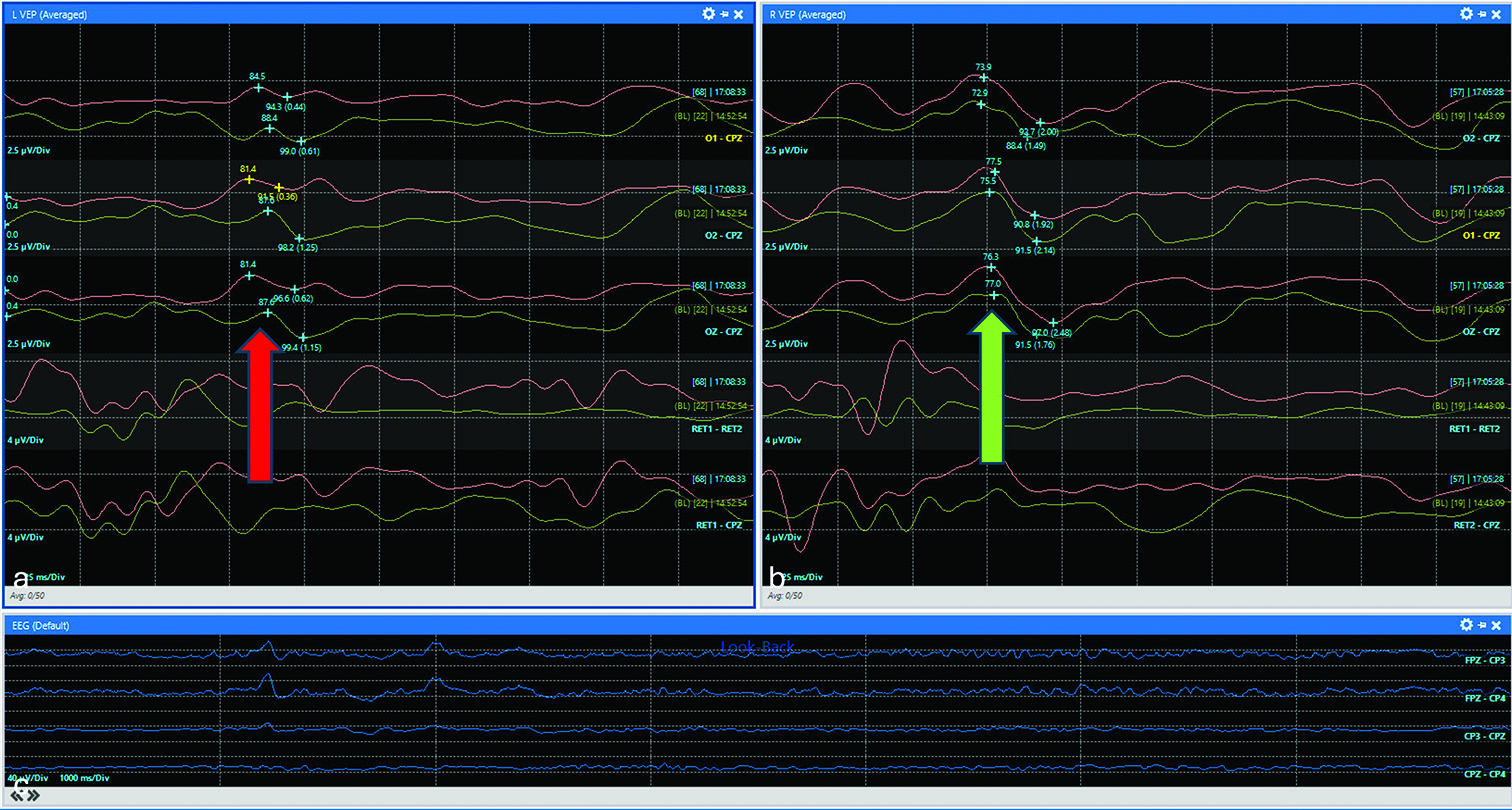
He was subsequently taken to the operating room for this procedure. Routine surgical adjuncts were utilized, including 3-D endoscopy, intraoperative neuronavigation, and VEPs with electroretinogram (ERG) and scalp electroencephalogram (EEG). The patient’s ERG responses were well formed and reproducible, but baseline VEP signals were not robust. This was not unexpected given the preoperative visual field deficits, and monitoring continued using these baselines. A low-to-moderate flow cerebrospinal fluid (CSF) leak was encountered intraoperatively, which was repaired with an inlay of abdominal fat, followed by a pedicled nasoseptal flap. During the packing of the fat into the sella, there was a transient decrease in the amplitude of the VEPs, and the surgical team was alerted immediately by the neuromonitoring team [
Figure 2:
Decrease in VEPs during fat packing. Red and green tracings represent current and baseline waveforms, respectively. (a) Representative VEPs from the left eye demonstrated decreased amplitude with no definitive peak in the red tracing compared to baseline (0.62 vs. 1.15 µV), best illustrated in the OZ-CPZ channel (red arrow). (b) In contrast, VEPs from the right eye showed no amplitude changes compared to the baseline (green arrow). (c) Continuous electroencephalogram monitoring. Visual-evoked potentials (VEP).
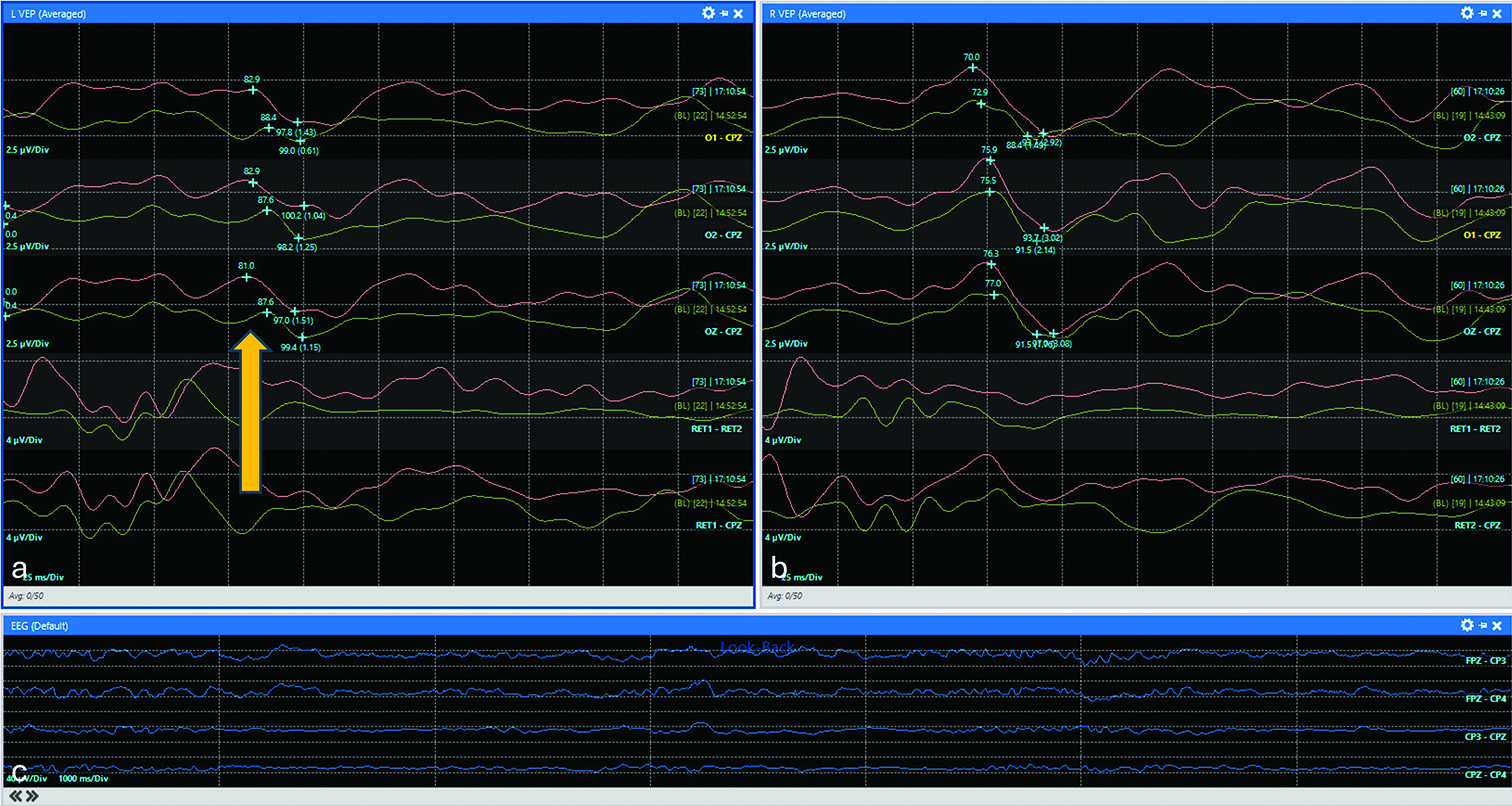
Figure 3:
Apparent improvement of VEPs after fat removal. Red and green tracings represent current and baseline waveforms, respectively. (a) Representative VEPs from the left eye demonstrated improved amplitude in the red tracing compared to baseline (1.51 vs. 1.15 µV) but with paradoxically shortened latencies (81.0 vs. 87.6 ms) (yellow arrow). (b) In contrast, VEPs from the right eye remained stable. (c) Continuous electroencephalogram monitoring demonstrated persistent activity that may have contributed to potential noise contribution. Visual-evoked potentials (VEP).
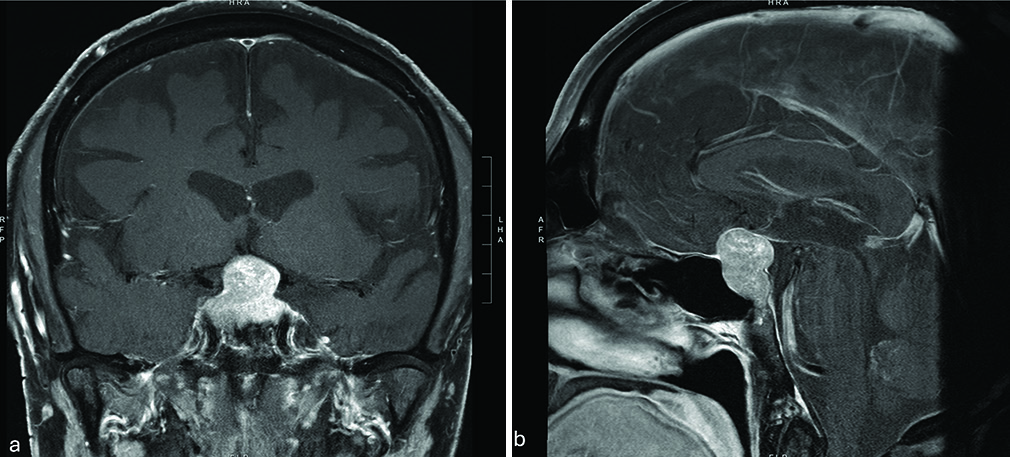
Postoperatively, the patient awoke grossly able to count fingers. As the anesthesia wore off, he thought the vision in his left eye had possibly worsened but was not sure, given that he had not taken his glaucoma eye drops. By the next morning, however, he was confident that his peripheral vision in his left eye had worsened and on examination, his temporal field deficit of the left eye had extended nasally toward complete midline. An ophthalmology consult was obtained, and the left visual field cut was confirmed. Otherwise stable visual acuity of 20/25 was noted on the right and with a stable visual acuity of 20/400 on the left. A stat MRI was obtained demonstrating expected postoperative changes with fat packing in the sella as well as the mass effect on the optic nerves and chiasm [
DISCUSSION
VEPs were first described during intra-orbital surgery in 1973, followed shortly by the first report during surgery around the sellar region in 1976.[
Several adjuncts may be useful to optimize the robustness of the VEP signal. First, the use of ERG in conjunction with intraoperative VEPs is useful as its presence establishes adequate stimulation of the retina and may avoid false positive results. Previous studies have suggested that a loss of signal may indicate a technical problem that needs to be rectified before VEPs can be reliably interpreted.[
Taken together, several studies looking at the use of VEPs in patients undergoing transsphenoidal surgery for pituitary adenomas have demonstrated that a combination of using LED goggles with simultaneous ERG monitoring as well as TIVA for anesthesia may optimize the robustness of the VEP waveform.[
The patient in our case had pre-existing visual field defects and poorly formed VEPs at baseline during the first surgery. Although anesthesia was optimized and TIVA was used, the signals were not robust and somewhat variable. Nonetheless, a critical insult was correctly identified during the surgery when overpacking with fat resulted in a decrease in the VEP amplitude. However, since the patient awoke with a deficit, it is questionable whether the observed amplitude improvement following the corrective removal of some fat during the first surgery was a true return to the signal baseline. In retrospect, the unusual paradoxical finding of decreased latencies may have indicated that noise intrusions occurred, potentially from high levels of EEG activity, that simulated a VEP signal with a peak at an earlier time, giving the false impression of restored signal amplitudes. During the second surgery, signals were so deteriorated that they were variable from the beginning and could not be monitored reliably. In the future, various strategies to minimize noise disruption from EEG, such as the use of burst suppression, may improve the accuracy of the VEP signal and, thus, produce more reliable amplitude and latency readings after surgical manipulation. In addition, others have described alternative methods to monitor the integrity of the optic nerves, such as direct epidural electrical stimulation of the optic nerves that could be considered in cases where there is bony exposure overlying the optic canals.[
During endoscopic transsphenoidal surgery, there are various strategies to reconstruct the skull base in the setting of an intraoperative CSF leak. At our institution, we routinely harvest an abdominal fat graft to pack the resection cavity, followed by a nasoseptal flap, particularly for high-flow CSF leaks. In this case, we encountered a low to moderate flow CSF leak (Esposito Grade 2) during the gentle peeling off of the tumor from the diaphragm.[
In our institutional experience spanning many years utilizing VEPs for all endoscopic transsphenoidal cases, we have found them to be helpful in demonstrating improvement of VEP signals after tumor resection, confirming adequate optic nerve decompression. In addition, in a handful of instances, transient decreases in VEPs have alerted us to overpacking of the resection cavity, which we rectified intraoperatively without any clinical consequences. However, as this case demonstrates, VEPs need to be interpreted with caution in patients with pre-existing pathological conditions of the visual apparatus.
CONCLUSION
While the general principle of intraoperative monitoring, which states that each patient is under his or her control, still holds for VEPs, the reproducibility of the signals needs to be carefully assessed. Burst suppression may be considered to minimize noise intrusions from EEG activity that may contribute to the precision of VEP signals. Unexpected findings, like the shortening of the VEP latencies in our case, should prompt further troubleshooting and discussion between the surgical, anesthesia, and neuromonitoring teams, as they may represent meaningful signal changes.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Backner Y, Petrou P, Glick-Shames H, Raz N, Zimmermann H, Jost R. Vision and vision-related measures in progressive multiple sclerosis. Front Neurol. 2019. 10: 455
2. Barbano L, Ziccardi L, Parisi V. Correlations between visual morphological, electrophysiological, and acuity changes in chronic non-arteritic ischemic optic neuropathy. Graefes Arch Clin Exp Ophthalmol. 2021. 259: 1297-308
3. Bosnjak R, Benedicic M. Direct epidural electrical stimulation of the optic nerve: A new method for intraoperative assessment of function. J Neurosurg. 2008. 109: 647-53
4. Bosnjak R, Benedicic M, Vittori A. Early outcome in endoscopic extended endonasal approach for removal of supradiaphragmatic craniopharyngiomas: A case series and a comprehensive review. Radiol Oncol. 2013. 47: 266-79
5. Bowman R, Walters B, Smith V, Prise KL, Handley SE, Green K. Visual outcomes and predictors in optic pathway glioma: A single centre study. Eye (Lond). 2023. 37: 1178-83
6. Cedzich C, Schramm J. Monitoring of flash visual evoked potentials during neurosurgical operations. Int Anesthesiol Clin. 1990. 28: 165-9
7. Cedzich C, Schramm J, Mengedoht CF, Fahlbusch R. Factors that limit the use of flash visual evoked potentials for surgical monitoring. Electroencephalogr Clin Neurophysiol. 1988. 71: 142-5
8. Chacko AG, Babu KS, Chandy MJ. Value of visual evoked potential monitoring during trans-sphenoidal pituitary surgery. Br J Neurosurg. 1996. 10: 275-8
9. Chi OZ, Field C. Effects of isoflurane on visual evoked potentials in humans. Anesthesiology. 1986. 65: 328-30
10. Chung SB, Park CW, Seo DW, Kong DS, Park SK. Intraoperative visual evoked potential has no association with postoperative visual outcomes in transsphenoidal surgery. Acta Neurochir (Wien). 2012. 154: 1505-10
11. Clauser LC, Tieghi R, Galie M, Franco F, Carinci F. Surgical decompression in endocrine orbitopathy. Visual evoked potential evaluation and effect on the optic nerve. J Craniomaxillofac Surg. 2012. 40: 621-5
12. Dotto PF, Berezovsky A, Sacai PY, Rocha DM, Fernandes AG, Salomao SR. Visual function assessed by visually evoked potentials in adults with orbital and other primary intracranial tumors. Eur J Ophthalmol. 2021. 31: 1351-60
13. Elsaid MA, Soliman S, Hashem O. Changes in the parameters of visual evoked potentials in media opacities. Clin Ophthalmol. 2023. 17: 3261-70
14. Esposito F, Dusick JR, Fatemi N, Kelly DF. Graded repair of cranial base defects and cerebrospinal fluid leaks in transsphenoidal surgery. Oper Neurosurg (Hagerstown). 2007. 60: 295-303 discussion 303-294
15. Feng R, Schwartz J, Loewenstern J, Kohli K, Lenina S, Ultakan S. The predictive role of intraoperative visual evoked potentials in visual improvement after endoscopic pituitary tumor resection in large and complex tumors: Description and validation of a method. World Neurosurg. 2019. 126: e136-43
16. Gaynor BG, Benveniste RJ, Lieberman S, Casiano R, Morcos JJ. Acellular dermal allograft for sellar repair after transsphenoidal approach to pituitary adenomas. J Neurol Surg B Skull Base. 2013. 74: 155-9
17. Gutzwiller EM, Cabrilo I, Radovanovic I, Schaller K, Boex C. Intraoperative monitoring with visual evoked potentials for brain surgeries. J Neurosurg. 2018. 130: 654-60
18. Hariharan P, Balzer JR, Anetakis K, Crammond DJ, Thirumala PD. Electrophysiology of olfactory and optic nerve in outpatient and intraoperative settings. J Clin Neurophysiol. 2018. 35: 3-10
19. Hayashi H, Kawaguchi M. Intraoperative monitoring of flash visual evoked potential under general anesthesia. Korean J Anesthesiol. 2017. 70: 127-35
20. Jashek-Ahmed F, Cabrilo I, Bal J, Sanders B, Grieve J, Dorward NL. Intraoperative monitoring of visual evoked potentials in patients undergoing transsphenoidal surgery for pituitary adenoma: A systematic review. BMC Neurol. 2021. 21: 287
21. Jo YJ, Kim HK, Lee JS. The clinical efficacy of preoperative flash visual evoked potential (VEP) for mature cataracts without a response to pattern VEP. Graefes Arch Clin Exp Ophthalmol. 2024. 262: 2525-32
22. Kamio Y, Sakai N, Sameshima T, Takahashi G, Koizumi S, Sugiyama K. Usefulness of intraoperative monitoring of visual evoked potentials in transsphenoidal surgery. Neurol Med Chir (Tokyo). 2014. 54: 606-11
23. Kodama K, Goto T, Sato A, Sakai K, Tanaka Y, Hongo K. Standard and limitation of intraoperative monitoring of the visual evoked potential. Acta Neurochir (Wien). 2010. 152: 643-8
24. Leng LZ, Brown S, Anand VK, Schwartz TH. “Gasket-seal” watertight closure in minimal-access endoscopic cranial base surgery. Neurosurgery. 2008. 62: ONSE342-3 discussion ONSE343
25. Luo Y, Regli L, Bozinov O, Sarnthein J. Clinical utility and limitations of intraoperative monitoring of visual evoked potentials. PLoS One. 2015. 10: e0120525
26. Mattogno PP, D’Alessandris QG, Rigante M, Granata G, Di Domenico M, Perotti V. Reliability of intraoperative visual evoked potentials (iVEPs) in monitoring visual function during endoscopic transsphenoidal surgery. Acta Neurochir (Wien). 2023. 165: 3421-9
27. Nakagawa I, Hidaka S, Okada H, Kubo T, Okamura K, Kato T. Effects of sevoflurane and propofol on evoked potentials during neurosurgical anesthesia. Masui. 2006. 55: 692-8
28. Nishimura F, Wajima D, Park YS, Motoyama Y, Nakagawa I, Yamada S. Efficacy of the visual evoked potential monitoring in endoscopic transnasal transsphenoidal surgery as a real-time visual function. Neurol India. 2018. 66: 1075-80
29. Pathak KS, Amaddio MD, Scoles PV, Shaffer JW, Mackay W. Effects of halothane, enflurane, and isoflurane in nitrous oxide on multilevel somatosensory evoked potentials. Anesthesiology. 1989. 70: 207-12
30. Raudzens PA. Intraoperative monitoring of evoked potentials. Ann N Y Acad Sci. 1982. 388: 308-26
31. Samra SK, Vanderzant CW, Domer PA, Sackellares JC. Differential effects of isoflurane on human median nerve somatosensory evoked potentials. Anesthesiology. 1987. 66: 29-35
32. Sasaki T, Itakura T, Suzuki K, Kasuya H, Munakata R, Muramatsu H. Intraoperative monitoring of visual evoked potential: Introduction of a clinically useful method. J Neurosurg. 2010. 112: 273-84
33. Schumann P, Kokemuller H, Tavassol F, Lindhorst D, Lemound J, Essig H. Optic nerve monitoring. Craniomaxillofac Trauma Reconstr. 2013. 6: 75-86
34. Sebel PS, Ingram DA, Flynn PJ, Rutherfoord CF, Rogers H. Evoked potentials during isoflurane anaesthesia. Br J Anaesth. 1986. 58: 580-5
35. Tabacaru B, Stanca HT. Further advances in the diagnosis and treatment of Leber’s Hereditary Optic Neuropathy-a review. Rom J Ophthalmol. 2022. 66: 13-6
36. Toyama K, Wanibuchi M, Honma T, Komatsu K, Akiyama Y, Mikami T. Effectiveness of intraoperative visual evoked potential in avoiding visual deterioration during endonasal transsphenoidal surgery for pituitary tumors. Neurosurg Rev. 2020. 43: 177-83
37. Uhl RR, Squires KC, Bruce DL, Starr A. Effect of halothane anesthesia on the human cortical visual evoked response. Anesthesiology. 1980. 53: 273-6
38. Uribe AA, Mendel E, Peters ZA, Shneker BF, Abdel-Rasoul M, Bergese SD. Comparison of visual evoked potential monitoring during spine surgeries under total intravenous anesthesia versus balanced general anesthesia. Clin Neurophysiol. 2017. 128: 2006-13
39. Wang H, Li F, Li J, Lin J, Liu M, Wang G. Electrophysiology as a prognostic indicator of visual recovery in diabetic patients undergoing cataract surgery. Graefes Arch Clin Exp Ophthalmol. 2021. 259: 1879-87
40. Wang X, Wang B, Cheng G, You Y, Tao C. Intradural fat graft packing is not indispensable in preventing postoperative cerebrospinal fluid leakage in endoscopic endonasal pituitary adenoma surgeries. Front Oncol. 2023. 13: 1222581
41. Wiedemayer H, Fauser B, Armbruster W, Gasser T, Stolke D. Visual evoked potentials for intraoperative neurophysiologic monitoring using total intravenous anesthesia. J Neurosurg Anesthesiol. 2003. 15: 19-24
42. Wilson WB, Kirsch WM, Neville H, Stears J, Feinsod M, Lehman RA. Monitoring of visual function during parasellar surgery. Surg Neurol. 1976. 5: 323-9
43. Wright JE, Arden G, Jones BR. Continuous monitoring of the visually evoked response during intra-orbital surgery. Trans Ophthalmol Soc U K (1962). 1973. 93: 311-4
44. Zamanipoor Najafabadi AH, Khan DZ, Muskens IS, Broekman ML, Dorward NL, van Furth WR. Trends in cerebrospinal fluid leak rates following the extended endoscopic endonasal approach for anterior skull base meningioma: A meta-analysis over the last 20 years. Acta Neurochir (Wien). 2021. 163: 711-9
45. Zhu H, Qiao N, Yang X, Li C, Ma G, Kang J. The clinical application of intraoperative visual evoked potential in recurrent craniopharyngiomas resected by extended endoscopic endonasal surgery. Clin Neurol Neurosurg. 2022. 214: 107149
46. Ziccardi L, Cioffi E, Barbano L, Gioiosa V, Falsini B, Casali C. Macular morpho-functional and visual pathways functional assessment in patients with spinocerebellar type 1 ataxia with or without neurological signs. J Clin Med. 2021. 10: 5271