- Department of Neurosurgery, Specialties Hospital of the 21st Century National Medical Center, Mexican Institute for Social Security, Mexico City, Mexico
- Department of Pathology, Specialties Hospital of the 21st Century National Medical Center, Mexican Institute for Social Security, Mexico City, Mexico
Correspondence Address:
Jesús Eduardo Falcón Molina, Department of Neurosurgery, Hospital de Especialidades, Centro Médico Nacional Siglo XXI, IMSS, Mexico City, Mexico.
DOI:10.25259/SNI_902_2023
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jesús Eduardo Falcón Molina1, Isauro Lozano Guzmán1, Marco Antonio Rodríguez Florido2, Emmanuel Maciel Ramos1, Luis Alfonso Castillejo Adalid1, Marco Antonio Ascencio Montiel1. Mucosa-associated lymphoid tissue lymphoma of the dura mimicking meningioma: A case report. 28-Feb-2025;16:63
How to cite this URL: Jesús Eduardo Falcón Molina1, Isauro Lozano Guzmán1, Marco Antonio Rodríguez Florido2, Emmanuel Maciel Ramos1, Luis Alfonso Castillejo Adalid1, Marco Antonio Ascencio Montiel1. Mucosa-associated lymphoid tissue lymphoma of the dura mimicking meningioma: A case report. 28-Feb-2025;16:63. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13423
Abstract
BackgroundPrimary central nervous system lymphomas (PCNSLs) are relatively infrequent tumors and are usually high-grade and aggressive neoplasms. A small portion of PCNSLs are low-grade lymphomas and can involve the dura. Mucosa-associated lymphoid tissue (MALT) lymphoma of the dura is an extremely rare subtype with only case reports and series documented in the literature.
Case DescriptionA 65-year-old woman presented with a history of headaches followed by progressive left hemiparesis. Imaging studies showed an extra-axial dural-based tumor causing midline shift. Gross total resection was achieved, and the patient was discharged without postoperative complications. Histopathological examination confirmed the diagnosis of MALT lymphoma of the dura. The patient was evaluated by the oncologist and received adjuvant chemotherapy. At the 10-month follow-up, the patient experienced remission of her symptoms, and the last magnetic resonance imaging showed no evidence of tumor recurrence.
ConclusionMALT lymphoma of the dura diagnosis requires a high level of suspicion because it can often mimic meningioma. Given its rarity, there is no consensus on the standard treatment strategy. Gross total resection followed by adjuvant therapy is an accepted treatment to manage these cases.
Keywords: Dura, Meningioma, Mucosa-associated lymphoid tissue lymphoma
INTRODUCTION
Primary central nervous system lymphoma (PCNSL) is a rare form of extranodal non-Hodgkin lymphoma that can develop in the brain, spine, cerebrospinal fluid, and eyes.[
Marginal zone-B lymphoma (MZBL) is a low-grade subtype that accounts for 7% of all non-Hodgkin lymphomas.[
LITERATURE REVIEW
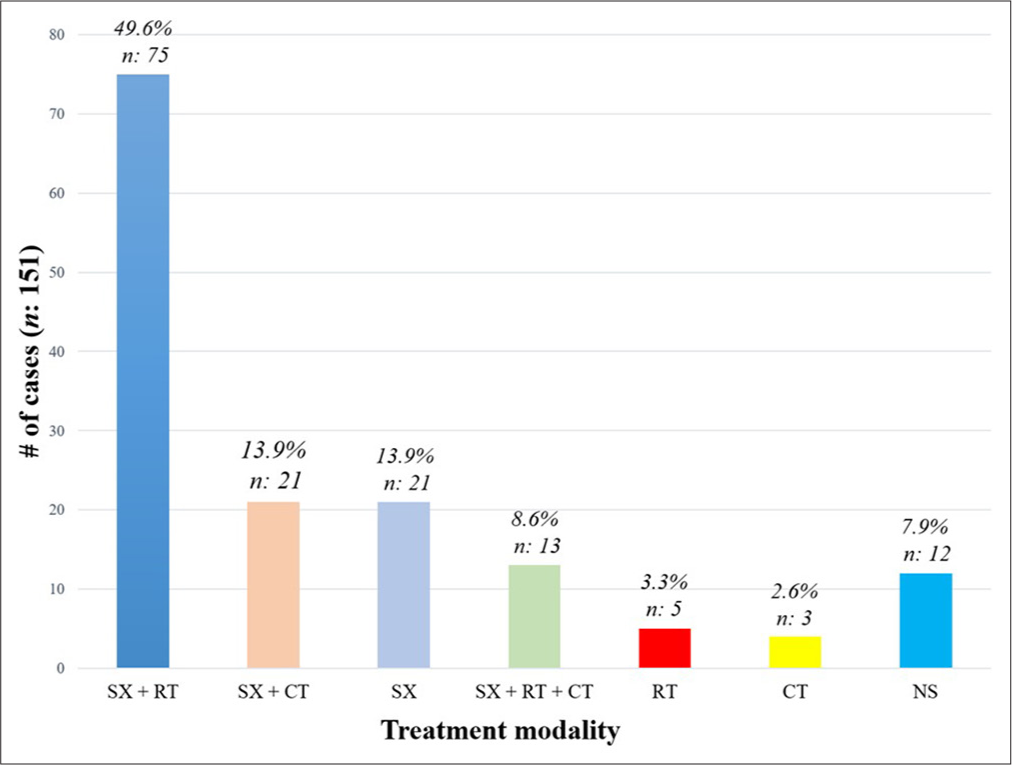
We conducted a literature search of articles written in English using PubMed from inception to 2024. Search terms included: ((dural) OR (meningeal)) AND ((mucosa-associated) OR (marginal zone lymphoma) OR (malt)). This search yielded 48 articles representing 179 cases of MALT lymphomas involving the dura. From this, we only included 151 cases in which intracranial involvement was described. Demographic data, tumor location, and treatment modality were collected. Information about treatment was divided into: only surgery, surgery + radiotherapy (RT), surgery + chemotherapy (CT), surgery + CT + RT, only RT, only CT, and not specified. Statistical analysis was performed using the software Statistical Package for the Social Sciences (version 25.0; IBM Corporation, Armonk, New York, USA).
RESULTS
We found 151 cases of MALT lymphomas involving intracranial dura. There was a prevalence of females (74.8%; n: 113) over males (25.1%; n: 38), and the mean age was 53.02 (±11.7) years. In order of frequency, the convexity was the most affected area (68%, n: 102), followed by tentorium (9.9%; n: 15), cavernous sinus (7.2%; n: 11), falx (5.2%, n: 8), and posterior fossa (3.9%; n: 6). There were other uncommon sites such as sellar/suprasellar region, sphenoid wing or clivus (5.9%; n: 9).
We found a wide spectrum of treatment modalities involving one or more procedures. The most frequent treatment was surgery + RT (49.6%; n: 75), followed by surgery + CT (13.9%; n: 21), only surgery (13.9%; n: 21), surgery + RT + CT (8.6%; n: 13), only RT (3.3%; n: 5), and only CT (2.6%; n: 3). In 12 cases, the modality of treatment was not specified (7.9%) [
CASE PRESENTATION
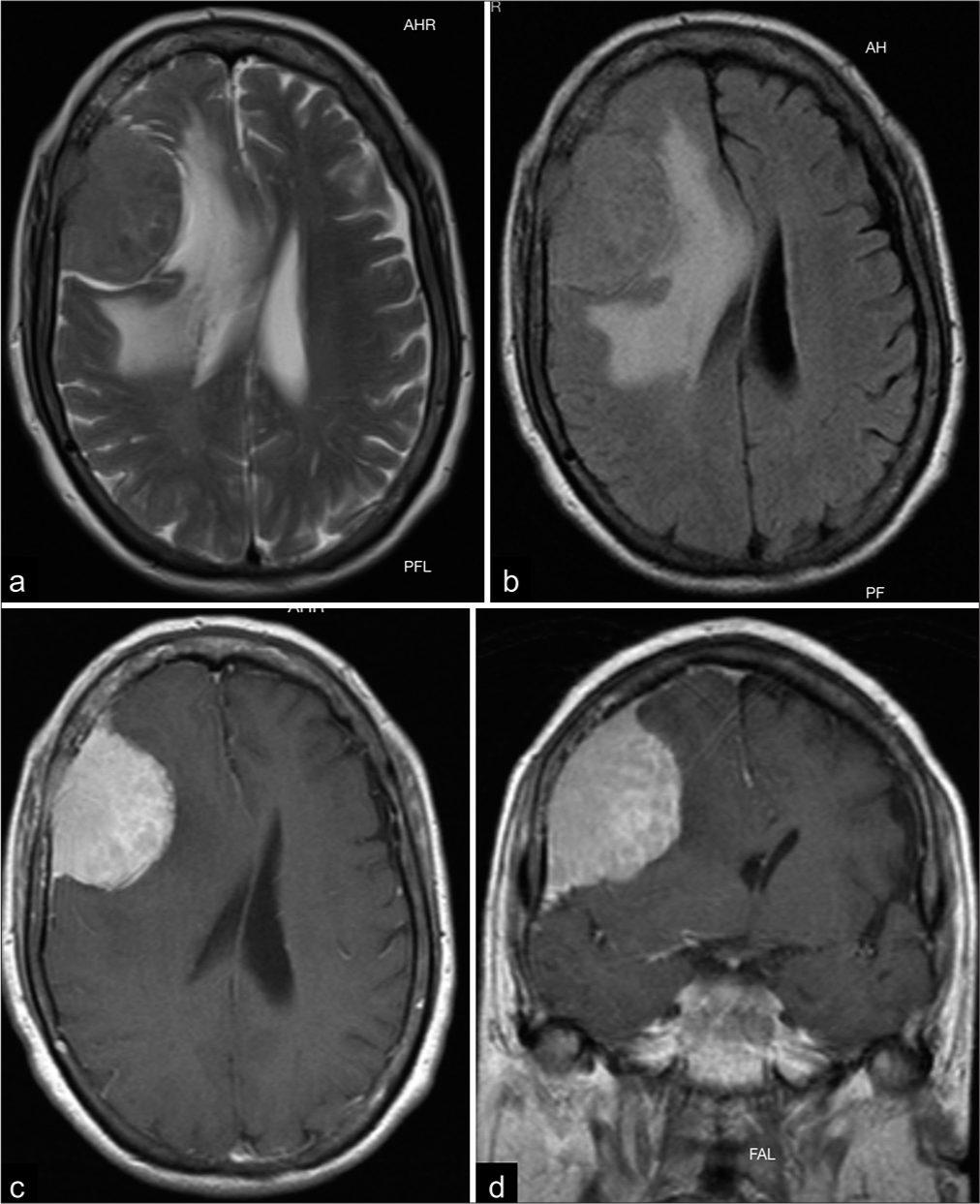
A 65-year-old female with a medical history of hypertension and diabetes presented with a 3-month history of asthenia, adynamia, and disorientation. She also experienced progressive left hemiparesis and worsening headaches during the past month. On clinical examination, dense left-sided hemiparesis was revealed, and pyramidal signs were present. Cranial magnetic resonance imaging (MRI) demonstrated a right extra-axial dural-based lesion arising from frontal convexity with perilesional edema and mass effect on the adjacent brain parenchyma [
Figure 2:
Preoperative magnetic resonance imaging. (a and b) Both T2-weighted imaging and T2 fluid-attenuated inversion recovery (FLAIR) images demonstrate a remarkable mass effect on the surrounding brain and vasogenic edema. (c and d) Axial T1-weighted and coronal T1-weighted images showing a right frontal extra-axial lesion with homogeneous and avid enhancement.
Surgical resection
The patient underwent surgery in a supine position with the head rotated to the left in three-point fixation in the Mayfield clamp. A Falconer incision was performed, followed by a right frontoparietotemporal craniotomy. A soft brownish tumor attached to the dura was resected. Hemostasis was secured, and the dura was closed with a dural graft. Bony infiltration was observed, and a cranioplasty with methyl methacrylate was performed. The postoperative course was unremarkable, and the patient was discharged on the 3rd postoperative day.
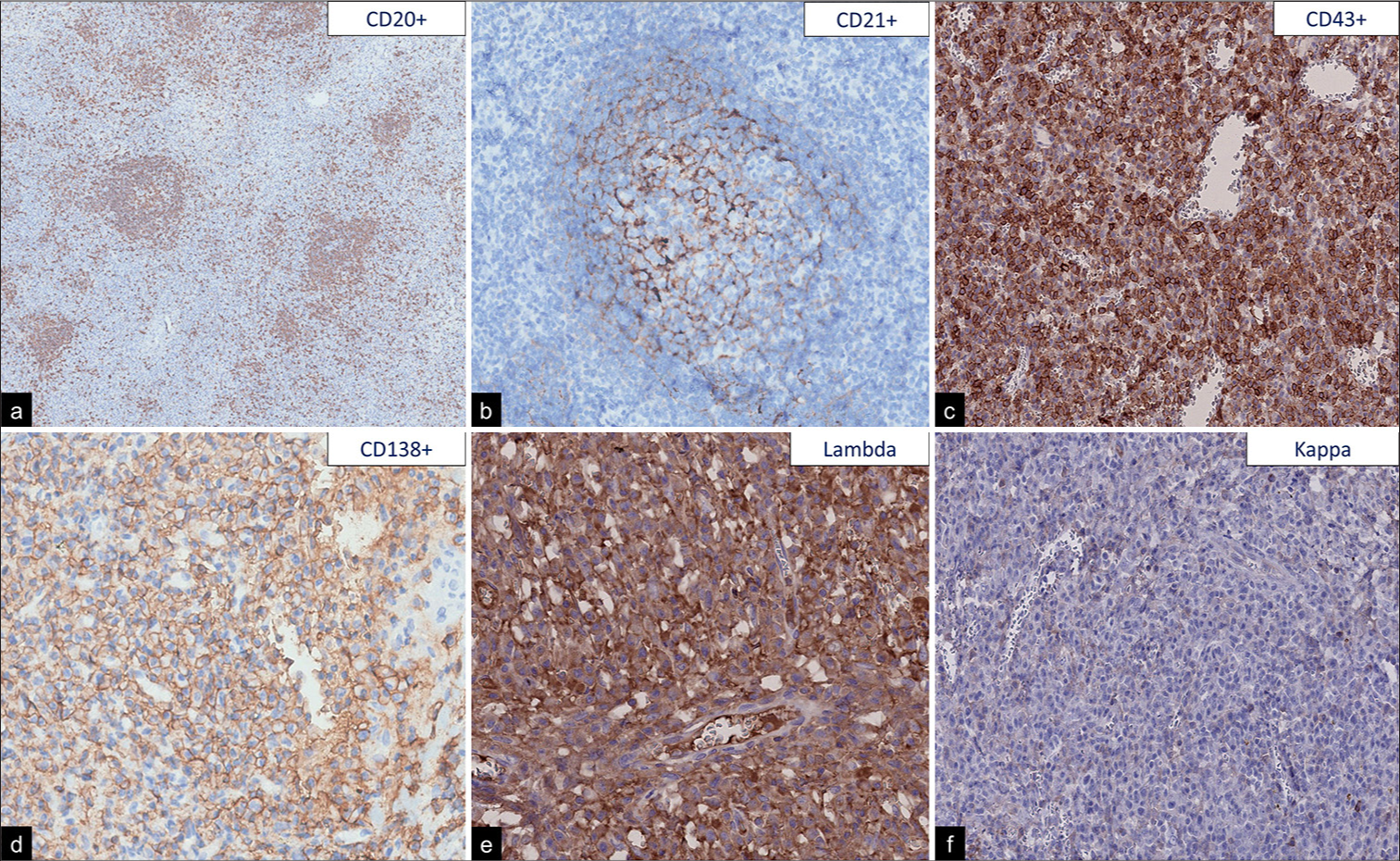
The neuropathology team received a specimen characterized by a nodular tumor attached to the dura that showed a brown-tan external surface with small vessels [
The patient was evaluated by the oncologist for potential adjuvant treatment. Four courses (every 3 weeks) of intravenous methotrexate-cytarabine combination therapy were administered: methotrexate 3.5 g/m2 on day 1 and cytarabine 2 g/m2 on days 2 and 3; repeated treatment. The patient underwent a whole-body positron emission tomography–computed tomography scan without evidence of an extracranial tumor location. At 10 months after surgery, she experienced improvement of hemiparesis, and the last MRI demonstrated no evidence of tumor recurrence [
DISCUSSION
PCNSLs represent 4-6% of extranodal lymphomas.[
The pathological features of MZBL arising in the CNS are similar to those of MZBL in other extranodal sites. They are composed of small or medium size lymphocytes and marginal zone cells, sometimes with remnants of reactive follicles with follicular colonization. Neoplastic cells show expression of pan-B cell markers (CD20, CD79a) and lack expression of CD5, CD10, CD23, BCL6, and cyclin D1.[
MALT lymphomas of the dura have a female predilection with a female-to-male ratio of 3:1. The median age at presentation was approximately 51 years (range: 28–77 years).[
In approximately 2% of cases, the imaging finding of an extra-axial dural-based lesion with homogeneous contrast enhancement on MRI is not a meningioma. The main differential diagnoses are metastases, solitary fibrous tumors, or lymphoproliferative diseases.[
The standard management for dural MALT lymphomas is not clearly defined. Due to their rarity, they are not usually included in most of the guidelines for non-gastric MZBL treatment.[
Patients with MALT lymphomas of the dura have a better prognosis compared to other extranodal sites. In a recent review, encompassing 93 cases reported that the 5-year overall survival and progression free-survival were 96.7% and 81.2%, respectively. Strict clinical and radiological follow-up is necessary because disease recurrence has been reported between 12 and 40 months after surgery.[
CONCLUSION
MALT lymphoma of the dura is a rare variant of PCNSL and usually presents as a dural-based lesion misdiagnosed as meningioma. The pathological diagnosis is made with the finding of small lymphocytes with diffuse patterns and immunohistochemical expression of B-cell markers. There is no standard management defined for this entity, but surgical treatment should be the first therapeutic step when a mass effect occurs, followed by adjuvant treatment.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Alderuccio JP, Kahl BS. Current treatments in marginal zone lymphoma. Oncology (Williston Park). 2022. 36: 206-15
2. Ayanambakkam A, Ibrahimi S, Bilal K, Cherry MA. Extranodal marginal zone lymphoma of the central nervous system. Clin Lymphoma Myeloma Leuk. 2018. 18: 34-7.e8
3. Bustoros M, Liechty B, Zagzag D, Liu C, Shepherd T, Gruber D. A rare case of composite dural extranodal marginal zone lymphoma and chronic lymphocytic leukemia/small lymphocytic lymphoma. Front Neurol. 2018. 9: 267
4. Cerhan JR, Habermann TM. Epidemiology of marginal zone lymphoma. Ann Lymphoma. 2021. 5: 1
5. Chihara D, Fowler NH, Oki Y, Fanale MA, Nastoupil LJ, Westin JR. Impact of histologic subtypes and treatment modality among patients with primary central nervous system lymphoma: A SEER database analysis. Oncotarget. 2018. 9: 28897-902
6. Grommes C, DeAngelis LM. Primary CNS lymphoma. J Clin Oncol. 2017. 35: 2410-8
7. Grommes C, Rubenstein J, DeAngelis LM, Ferrari AJ, Batchelor TT. Comprehensive approach to diagnosis and treatment of newly diagnosed primary CNS lymphoma. Neuro Oncol. 2019. 21: 296-305
8. Itoh T, Shimizu M, Kitami K, Kamata K, Mitsumori K, Fujita M. Primary extranodal marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue type in the CNS. Neuropathology. 2001. 21: 174-80
9. Karschnia P, Batchelor TT, Jordan JT, Shaw B, Winter SF, Barbiero FJ. Primary dural lymphomas: Clinical presentation, management, and outcome. Cancer. 2020. 126: 2811-20
10. La Rocca G, Auricchio AM, Mazzucchi EM, Lus T, Della GM, Altieri R. Intracranial dural based marginal zone MALT-type B-cell lymphoma: A case-Based update and literature review. Br J Neurosurg. 2023. 37: 1480-1486
11. Lopetegui N, Delasos L, Daniyal S, Kumar M, Harrison J. Primary central nervous system marginal zone B-cell lymphoma arising from the dural meninges: A case report and review of literature. Clin Case Rep. 2020. 8: 491-7
12. Nagai V, de Sousa LF, Alencar G, Tavares L, Correa G, Fontoura DJ. Dural-based lesions: Is it a meningioma?. Neuroradiology. 2021. 63: 1215-25
13. Ostrom QT, Price M, Neff C, Cioffi G, Waite KA, Kruchko C. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2016-2020. Neuro Oncol. 2023. 25: iv1-99
14. Pons A, Naval P, Velasco R, Vidal N, Majós C. Imaging of lymphomas involving the CNS: An update-review of the full spectrum of disease with an emphasis on the world health organization classifications of CNS tumors 2021 and hematolymphoid tumors 2022. AJNR Am J Neuroradiol. 2023. 44: 358-66
15. Razaq W, Goel A, Amin A, Grossbard ML. Primary central nervous system mucosa-associated lymphoid tissue lymphoma: Case report and literature review. Clin Lymphoma Myeloma. 2009. 9: E5-9
16. WHO Classification of Tumours Editorial Boa, editors. World Health Organization Classification of Tumours of the Central Nervous System. Lyon: International Agency for Research on Cancer; 2021. p.
17. Zucca E, Arcaini L, Buske C, Johnson PW, Ponzoni M, Raderer M. Marginal zone lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020. 31: 17-29