- Departments of Neurosurgery, Sendai City Hospital, 1-1-1, Asuto-Nagamachi, Taihaku-ku,
- Departments of Neurosurgery, Tohoku University Graduate School of Medicine, 1-1 Seiryo, Aobaku, Sendai, Japan.
Correspondence Address:
Hiroshi Karibe
Departments of Neurosurgery, Tohoku University Graduate School of Medicine, 1-1 Seiryo, Aobaku, Sendai, Japan.
DOI:10.25259/SNI-18-2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Masahito Katsuki, Ayumi Narisawa, Hiroshi Karibe, Motonobu Kameyama, Teiji Tominaga. Mutism resulting from heterochronic bilateral cerebellar hemorrhages – A case report. 25-Jun-2019;10:122
How to cite this URL: Masahito Katsuki, Ayumi Narisawa, Hiroshi Karibe, Motonobu Kameyama, Teiji Tominaga. Mutism resulting from heterochronic bilateral cerebellar hemorrhages – A case report. 25-Jun-2019;10:122. Available from: http://surgicalneurologyint.com/surgicalint-articles/9409/
Abstract
Background: Cerebellar mutism (CM) is a neurological condition characterized by lack of speech due to cerebellar lesions. Interruption of the bilateral dentatothalamocortical (DTC) pathways at midline structure seems the principal cause of CM but not fully understood. We described a rare case of CM due to heterochronic bilateral cerebellar hemorrhages.
Case Description: An 87-year-old woman presented with depression of alertness after sudden vomiting. Neurologically, mild dysmetria and mutism were observed. The head computed tomography (CT) showed both a fresh right cerebellar hemorrhage and an obsolete left one. The patient was diagnosed as CM since both the thalamus and the supplementary motor area were bilaterally intact on both CT and magnetic resonance imaging. Medical treatment and rehabilitation improved her ataxia and ambulation. She became cognitively alert and could communicate by nodding, shaking her head, or facial expression. However, her mutism did not change at 4 months after the stroke.
Conclusion: There are few reports on CM due to direct injuries to the bilateral dentate nuclei. Since our case did not show any injury other than bilateral dentate nuclei, this report can support the hypothesis that the interruptions of the bilateral DTC are the cause of CM.
Keywords: Cerebellar hemorrhage, Cerebellar mutism, Diaschisis, Thalamodentatocortical pathway
INTRODUCTION
Cerebellar mutism (CM) is a neurological condition characterized by lack of speech despite reading and writing with intact comprehension after cerebellar injury,[
Interruptions of the bilateral dentatothalamocortical pathways (DTC) may be the principal cause of CM.[
We report a CM patient with both a recent right cerebellar hemorrhage and an older left one. The head computed tomography (CT) or magnetic resonance imaging (MRI) showed injuries to the bilateral dentate nuclei. Previous reports are scarce on CM resulting from injuries of the bilateral dentate nuclei.[
CASE REPORT
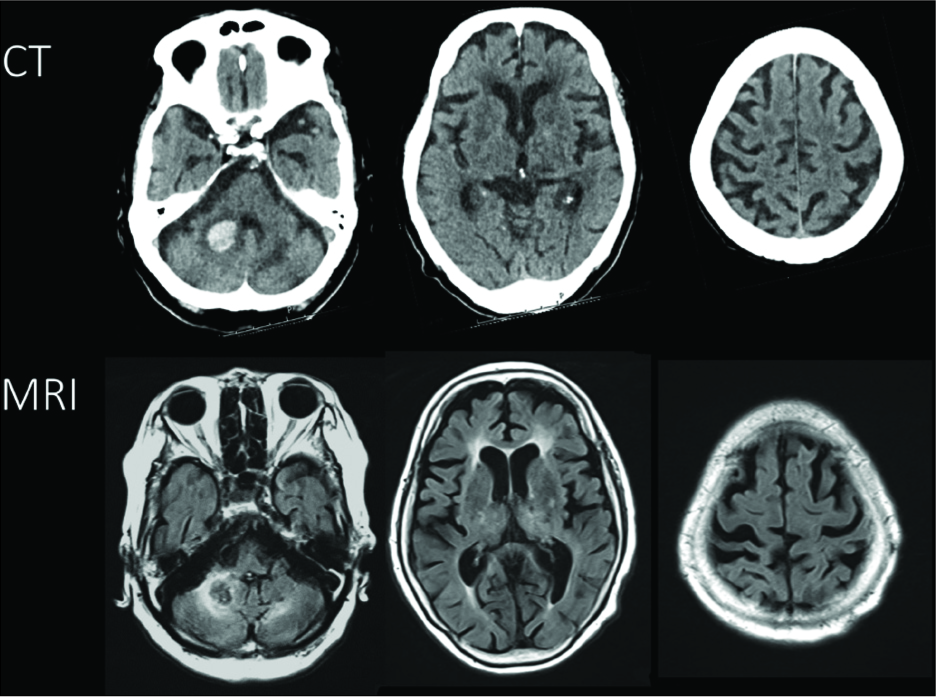
An 87-year-old right-handed female patient presented to our emergency room with depression of alertness after sudden vomiting. She had suffered from the left cerebellar hemorrhage 20 years ago and had been taking antihypertensive medications. Her blood pressure was 235/135 mmHg on admission. She had mild dysmetria and did not speak any words although could obey commands, with the Glasgow coma scale score of 11 (E4V1M6). The head CT showed a high-density area in the right cerebellar hemisphere, presenting 9 mL of a right cerebellar hematoma. It also showed a low-density area in the left cerebellar hemisphere, presenting an older left cerebellar hemorrhage. The head MRI showed perifocal edema surrounding the right cerebellar hematoma. Both the thalamus and the supplementary motor area were bilaterally intact on both CT and MRI [
Figure 1
Computed tomography showed a high-density area in the right cerebellar hemisphere and a low-density area in the left cerebellar hemisphere (upper row). The head magnetic resonance imaging showed edema around the right cerebellar hematoma (lower row). Both the thalamus and the supplementary motor area were bilaterally intact on both the computed tomography and magnetic resonance imaging images.
On the following day, her neurological findings did not change and rehabilitation began. The CT did not show hematoma expansion. On the 3rd day, she was mute but cognitively alert, although she could communicate by nodding, shaking her head, or facial expression enabled communication.
She could not walk independently and was transferred to a rehabilitation hospital 31 days after the onset of the right cerebellar hemorrhage. Her mutism improved a bit and a few short words (e.g. yes and no) were uttered. Four months after the onset, she could walk with a little care. However, her mutism did not change compared to when she was transferred to the rehabilitation hospital. The CT and MRI did not show any additional findings rather than the injuries of the bilateral dentate nuclei. She was diagnosed as CM caused by heterochronic bilateral cerebellar hemorrhages.
DISCUSSION
In general, mutism is defined as the inability to speak despite cognitively alert patient. It can occur due to lesions in several parts of the brain, such as Broca’s area, the supplementary motor area, thalamus, mesencephalic reticular formation regions, and bilateral hemispheric lesions.[
CM is one of the types of transient mutism resulting from interruptions of the bilateral DTC pathways, in general. Posterior fossa tumor surgery is the most common cause of this syndrome, as symptoms usually last for a few months. Surgical excision of the posterior fossa tumor causes CM, occurring in 27.7%[
The anatomical substrate of CM is not fully understood. Vermis, brainstem, and anywhere along the DTC are reported as a candidate for responsible regions of CM after posterior fossa surgery.[
CM seems to be also related to brainstem involvement of the tumor.[
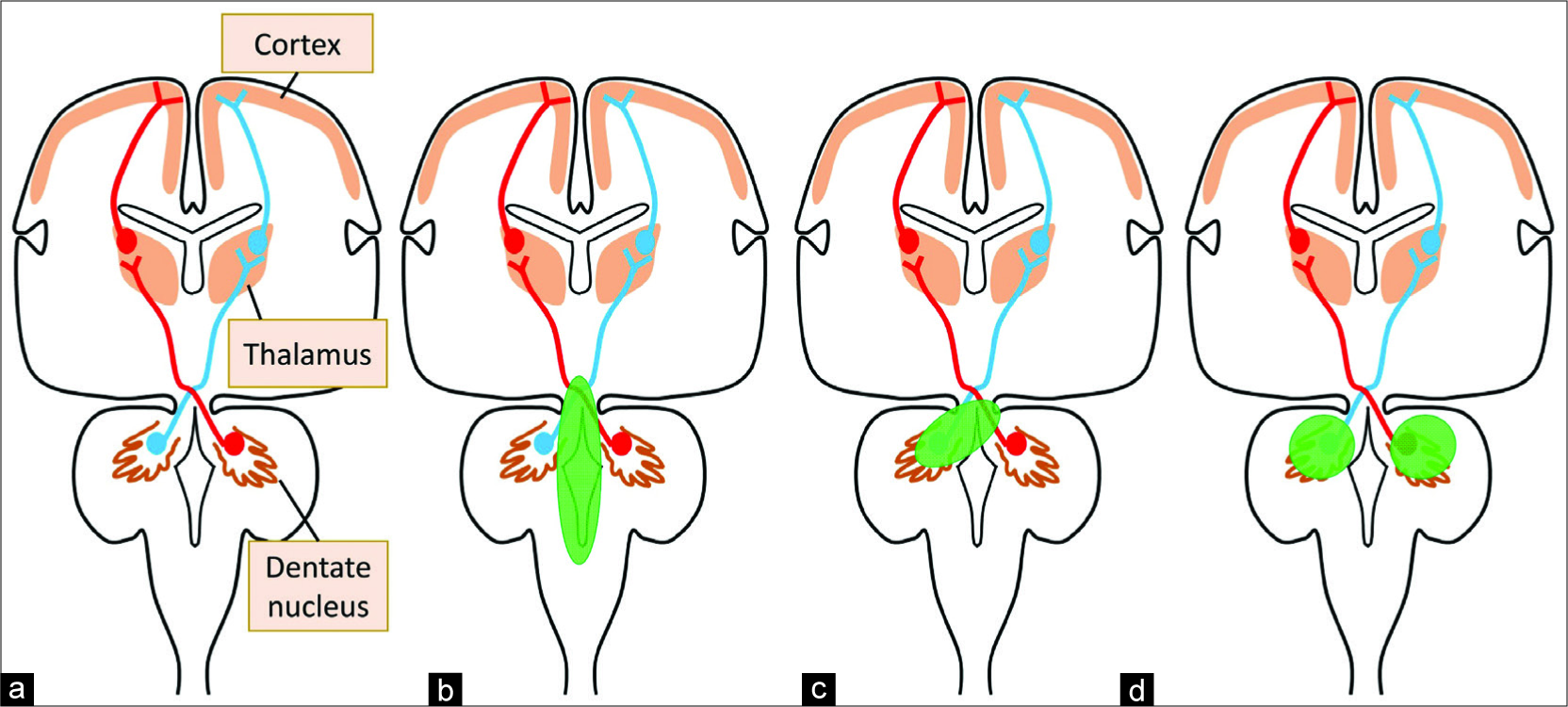
Figure 2
Schematic illustration of the dentatothalamocortical pathway. (a) Surgical excision of posterior fossa tumor would damage the brainstem directly or cause postoperative edema in the brainstem. (b) Massive cerebellar hemorrhage or surgical intervention would injure both the unilateral dentate nucleus and the brainstem or the contralateral superior cerebellar peduncle. (c) Our case presented lesions to the bilateral dentate nuclei which interrupt the dentatothalamocorticals bilaterally. (d) All the lesions interrupt the dentatothalamocortical bilaterally, and cerebellar mutism would be caused.
There have been six previous case reports of CM after cerebellar hemorrhage [
In the present case, injuries were limited in the bilateral dentate nuclei due to the recent right cerebellar hemorrhage and the older left cerebellar hemorrhage without surgical treatment. Besides, the CT and MRI did not show any other lesions along the DTC. In previous reports on CM, due to surgical intervention or massive hemorrhage, it has been difficult to indicate the responsible region for CM precisely. The present case seems simple and can support the theory that bilateral interruptions of the DTC are the cause of CM [
Some cases of CM resulting from injuries of bilateral dentate nuclei have been reported. Kubota et al. reported CM due to rotavirus-associated acute cerebellitis.[
CONCLUSION
We reported the patient with CM due to the recent right cerebellar hemorrhage and the older left one. Both CT and MRI did not show any lesions other than the bilateral dentate nuclei. This report can support the hypothesis that bilateral interruptions of the DTC are the cause of CM.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
Conception and design: Hiroshi Karibe. Drafting the article: all authors. Supervision: Hiroshi Karibe, Teiji Tominaga.
References
1. Ackermann H, Mathiak K, Riecker A. The contribution of the cerebellum to speech production and speech perception: Clinical and functional imaging data. Cerebellum. 2007. 6: 202-13
2. Catsman-Berrevoets CE, Aarsen FK. The spectrum of neurobehavioural deficits in the posterior fossa syndrome in children after cerebellar tumour surgery. Cortex. 2010. 46: 933-46
3. Coplin WM, Kim DK, Kliot M, Bird TD. Mutism in an adult following hypertensive cerebellar hemorrhage: Nosological discussion and illustrative case. Brain Lang. 1997. 59: 473-93
4. Dailey AT, McKhann GM, Berger MS. The pathophysiology of oral pharyngeal apraxia and mutism following posterior fossa tumor resection in children. J Neurosurg. 1995. 83: 467-75
5. De Smet HJ, Mariën P. Posterior fossa syndrome in an adult patient following surgical evacuation of an intracerebellar haematoma. Cerebellum. 2012. 11: 587-92
6. Gudrunardottir T, Sehested A, Juhler M, Schmiegelow K. Cerebellar mutism: Review of the literature. Childs Nerv Syst. 2011. 27: 355-63
7. Guidetti B, Fraioli B. Neurosurgical treatment of spasticity and dyskinesias. Acta Neurochir (Wien). 1977. 24: 27-39
8. Idiaquez J, Fadic R, Mathias CJ. Transient orthostatic hypertension after partial cerebellar resection. Clin Auton Res. 2011. 21: 57-9
9. Kariyattil R, Rahim MI, Muthukuttiparambil U. Cerebellar mutism following closed head injury in a child. Sultan Qaboos Univ Med J. 2015. 15: 133-5
10. Kawai H, Ohta F, Matsumoto Y, Yamamoto Y. An adult case of cerebellar mutism after removal of cerebellar hematoma. No To Shinkei. 2001. 53: 475-9
11. Koh S, Turkel SB, Baram TZ. Cerebellar mutism in children: Report of six cases and potential mechanisms. Pediatr Neurol. 1997. 16: 218-9
12. Kubota T, Suzuki T, Kitase Y, Kidokoro H, Miyajima Y, Ogawa A. Chronological diffusion-weighted imaging changes and mutism in the course of rotavirus-associated acute cerebellitis/cerebellopathy concurrent with encephalitis/ encephalopathy. Brain Dev. 2011. 33: 21-7
13. Lee S, Na YH, Moon HI, Tae WS, Pyun SB. Neuroanatomical mechanism of cerebellar mutism after stroke. Ann Rehabil Med. 2017. 41: 1076-81
14. Makarenko S, Singh N, McDonald PJ. Non-surgical transient cerebellar mutism-case report and systematic review. Childs Nerv Syst. 2018. 34: 535-40
15. Mariën P, Verslegers L, Moens M, Dua G, Herregods P, Verhoeven J. Posterior fossa syndrome after cerebellar stroke. Cerebellum. 2013. 12: 686-91
16. Nishikawa M, Komiyama M, Sakamoto H, Yasui T, Nakajima H. Cerebellar mutism after basilar artery occlusion case report. Neurol Med Chir (Tokyo). 1998. 38: 569-73
17. Reed-Berendt R, Phillips B, Picton S, Chumas P, Warren D, Livingston JH. Cause and outcome of cerebellar mutism: Evidence from a systematic review. Childs Nerv Syst. 2014. 30: 375-85
18. Tamburrini G, Frassanito P, Chieffo D, Massimi L, Caldarelli M, Di Rocco C. Cerebellar mutism. Childs Nerv Syst. 2015. 31: 1841-51
19. van Baarsen KM, Grotenhuis JA. The anatomical substrate of cerebellar mutism. Med Hypotheses. 2014. 82: 774-80