- Department of Neurosurgery, Cedars-Sinai Medical Center, Los Angeles, California, USA
- Department of Pathology, St. Vincent Medical Center, Los Angeles, California, USA
- Swedish Neuroscience Institute, Swedish Medical Center, Seattle, Washington, USA
- House Clinic, Los Angeles, California, USA
Correspondence Address:
Gregory Lekovic
House Clinic, Los Angeles, California, USA
DOI:10.4103/sni.sni_422_17
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Tridu R. Huynh, Conrad Lu, Doniel Drazin, Gregory Lekovic. Myxopapillary ependymoma with anaplastic features: A case report with review of the literature. 20-Sep-2018;9:191
How to cite this URL: Tridu R. Huynh, Conrad Lu, Doniel Drazin, Gregory Lekovic. Myxopapillary ependymoma with anaplastic features: A case report with review of the literature. 20-Sep-2018;9:191. Available from: http://surgicalneurologyint.com/surgicalint-articles/9018/
Abstract
Background:Myxopapillary ependymoma (MPE) with anaplastic features is extremely rare, with only three case reports in the literature.
Case Description:We report the case of a MPE with anaplastic features in a 24-year-old female who presented with a dominant lumbar mass along with intracranial and sacral metastases. Upon gross total resection of the dominant tumor located at L2-L3, it appeared to arise from the filum terminale, and had a solid component in addition to soft or necrotic areas. Histologically, the tumor was composed of the two classic components of MPE: (1) low-grade ependymal cells surrounding blood vessels, producing the papillary appearance and (2) perivascular myxoid material between blood vessels and ependymal cells, creating the myxopapillary appearance. The high-grade anaplastic component showed hypercellularity, brisk mitotic rate, and vascular proliferation, with frequent pleomorphic cells and atypical mitotic figures. It was positive for vimentin and glial fibrillary acidic protein (GFAP); negative for epithelial membrane antigen (EMA), CAM5.2, creatine kinase 7 (CK7), CK20; and the MIB-1 index (Ki-67) was 8–38%. Ten months after initial resection, follow-up magnetic resonance imaging revealed new lesions in (1) the hypothalamus, (2) the left pons, and (3) the left medial temporal lobe, which were treated with radiosurgery. Eight months later (18 months from initial surgery), the patient underwent thoracic laminectomy for a large leptomeningeal metastasis at T6 and T8.
Conclusion:The present case of MPE with anaplastic features is the fourth case on record in the medical literature.
Keywords: Anaplastic ependymoma, myxopapillary ependymoma, myxopapillary ependymoma with anaplastic features
INTRODUCTION
Ependymomas are primary central nervous system (CNS) tumors that arise from the ependymal cells lining the choroid plexus, the white matter adjacent to the angulated ventricles, and the central canal of the spinal cord. In the 2016 World Health Organization (WHO) classification of CNS tumors, ependymal tumors are divided into five major subtypes: myxopapillary ependymoma (MPE) and sub-ependymoma (grade I), classic ependymoma (grade II), RELA fusion-protein positive ependymoma (grade II or III), and anaplastic ependymoma (grade III).[
MPEs are usually benign, slow growing gliomas that have been primarily described in the conus-cauda equina-filum terminale region, though rare occurrences in the cervico-thoracic spinal cord, lateral ventricle, and brain parenchyma have been reported.[
Distant metastases to brain parenchyma and other organs have also been reported.[
Anaplasia in ependymomas is usually defined by the presence of anaplastic features, such as hypercellularity, frequent mitotic figures, pseudopalisading necrosis, vascular proliferation, and cellular as well as nuclear pleomorphism.[
We report the case of a MPE with anaplastic features in a 24-year-old female. Clinical, histopathological, and immunohistochemical findings are hereby described.
CASE REPORT
History and examination
A 24-year-old female presented with acute on chronic exacerbation of several months of back pain with new right radicular pain in the right thigh and lateral leg.
On physical examination, the patient was a well-developed, well-appearing young female in no acute distress. She did not have any cutaneous lesions, including axillary freckling, café au lait spots, or cutaneous neurofibromas. Her neurologic examination was unremarkable with intact nerve function; similarly, her motor examination was unremarkable with 5/5 motor strength in all extremities.
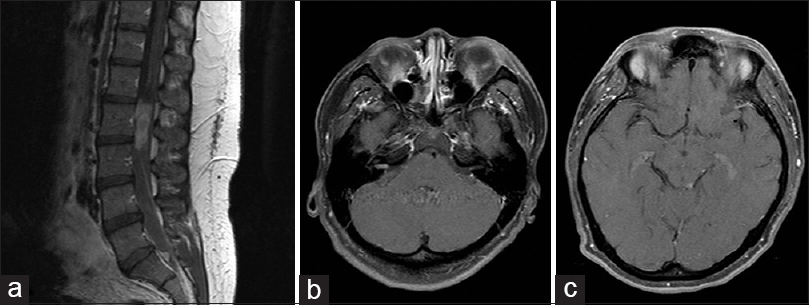
Magnetic resonance imaging (MRI) of the lumbar spine with and without contrast demonstrated an enhancing mass occupying the majority of the spinal canal from L2-L3 [
Figure 1
(a) Contrast enhanced sagittal T1-weighted magnetic resonance imaging demonstrates a homogeneously enhancing mass centered on L2-3 as well as a small sacra drop metastasis in the sacrum (arrowhead). Axial T1 contrast enhanced MRI demonstrates bilateral enhancement in the internal auditory canals, and a small enhancing focus was identified in the region of the left tectum (panels b and c, respectively)
Operation
A lumbar laminectomy at the level of L2-L3 was performed with gross total resection of the dominant lumbar tumor achieved. At surgery, the tumor appeared to be grossly necrotic, appeared to arise from the filum terminale, and had a solid component in addition to the soft or necrotic areas.
Histopathological examination
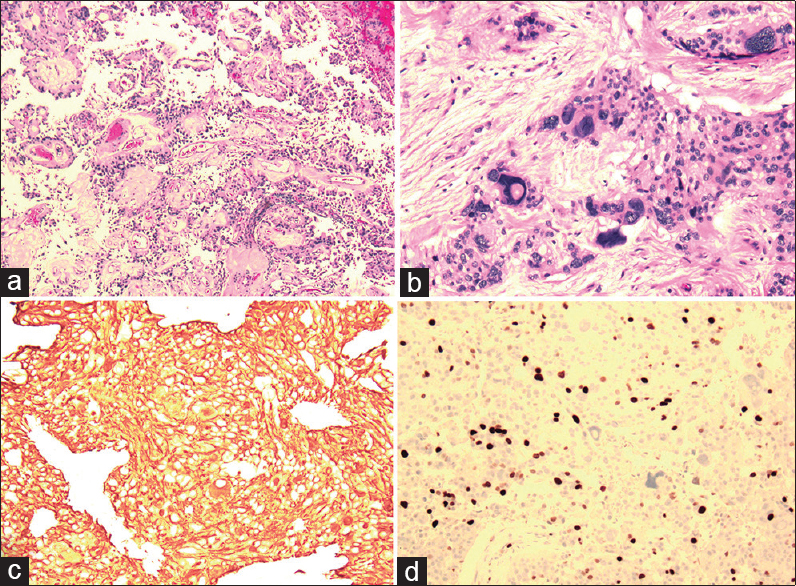
Low-grade areas of tumor [
Figure 2
Low magnification view (panel a) demonstrating typical low-grade myxopapillary ependymoma including papillary appearance and fibrovascular cores. Other areas of tumor (panel b, shown under high magnification) demonstrate anaplasia and cellular atypia typical of high-grade tumor. Tumor specimen was diffusely positive for GFAP staining, indicating a neuronal differentiation, and demonstrated a high Ki-67 index (panels c and d, respectively)
High-grade anaplastic areas of tumor [
The combination of areas of typical MPE-appearing tumor interspersed with areas of ependymoma with anaplastic or high-grade features was consistent with a diagnosis of MPE with anaplastic features.
Postoperative course
Postoperatively, the patient did well without any new neurologic deficits; bladder and bowel functions were intact, and the patient had full motor strength in bilateral lower extremities. Postoperative MRI confirmed gross total resection of the tumor.
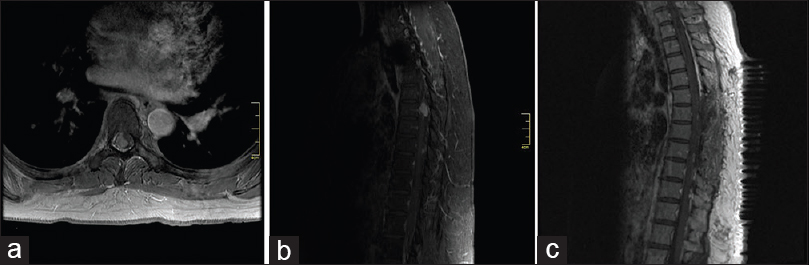
Given the presence of anaplastic or malignant-appearing tumor on histopathology, and the presence of disseminated metastases at presentation, craniospinal radiation was recommended. However, the patient refused, and proceeded with external beam radiation to the tumor bed in the lumbar spine only. Initially, she refused any treatment for her cranial metastases, until progression of intracranial disease was documented on follow-up MRI. Again, the patient refused craniospinal radiation, opting for stereotactic radiosurgery to the growing lesions. Ten months after her surgical resection, the patient therefore underwent CyberKnife radiosurgery to five intracranial lesions: (1) the lesion in the hypothalamus, (2) the lesion in the right internal auditory canal, (3) a new left pontine lesion, (4) the left tectum lesion, and (5) a new left medial temporal lesion. The hypothalamic lesion was treated with a dose of 25 gray in 5 fractions, and the remainder of the lesions were treated in 3 fractions of 8 gray each. The patient was followed with serial MRIs every 3 months and again demonstrated progression of both intracranial and spinal disease. At 18 months postoperation from her initial surgery, the patient underwent thoracic laminectomy for a large metastasis at T6 and T8 [
Figure 3
Axial and sagittal contrast enhanced T1-weighted MRI showing large enhancing mass within the thoracic spine causing compression of the thoracic spinal cord (a and b, respectively). Postoperative sagittal MRI demonstrates gross total resection of enhancing mass (panel c) MRI, magnetic resonance imaging
DISCUSSION
MPE with anaplastic features is a rare occurrence, with only three case reports in the literature [
The immunohistochemical description of MPE has been reported, which consist of positivity for GFAP and vimentin, consistent with the present case.[
Conventional MPE have a favorable overall survival, but despite their low-grade designation, almost half of all patients experience local recurrence (2–15 years after operation), irrespective of adequate excision.[
The 5-year survival rate of patients with anaplastic ependymoma, on the other hand, has been reported to be less than 20%. Radiotherapy following partial excision is then recommended.
Grading of ependymoma is currently difficult and of questionable clinical utility under the current WHO guidelines.[
CONCLUSIONS
MPE is a benign tumor that has the potential for malignant degeneration. Although MPE is a WHO grade 1 tumor, cerebrospinal fluid metastases at the time of initial diagnosis is well reported. In contrast, other features of malignancy, including high-grade anaplastic histology, are rare, but when occurring is associated with an aggressive clinical course similar to that of nonmyxopapillary (high grade) ependymoma. The management of patients with myxopapiallry ependymoma should take into account the possibility of malignant transformation/degeneration.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Akyurek S, Chang EL, Yu TK, Little D, Allen PK, McCutcheon I. Spinal myxopapillary ependymoma outcomes in patients treated with surgery and radiotherapy at M.D. Anderson Cancer Center. J Neurooncol. 2006. 80: 177-83
2. Ang LC, Taylor AR, Bergin D, Kaufmann JC. An immunohistochemical study of papillary tumors in the central nervous system. Cancer. 1990. 65: 2712-9
3. Awaya H, Kaneko M, Amatya VJ, Takeshima Y, Oka S, Inai K. Myxopapillary ependymoma with anaplastic features. Pathol Int. 2003. 53: 700-3
4. Beschorner R, Wehrmann M, Ernemann U, Bonin M, Horber V, Oehl-Jaschkowitz B. Extradural ependymal tumor with myxopapillary and ependymoblastic differentiation in a case of Schinzel-Giedion syndrome. Acta Neuropathol. 2007. 113: 339-46
5. Chakraborti S, Kini H, Pai KG, Upadhyaya V. Sacrococcygeal myxopapillary ependymoma with anaplastic ependymoma component in an infant. J Pediatr Neurosci. 2012. 7: 218-20
6. Chi JH, Cachola K, Parsa AT. Genetics and molecular biology of intramedullary spinal cord tumors. Neurosurg Clin N Am. 2006. 17: 1-5
7. Coffin CM, Swanson PE, Wick MR, Dehner LP. An immunohistochemical comparison of chordoma with renal cell carcinoma, colorectal adenocarcinoma, and myxopapillary ependymoma: A potential diagnostic dilemma in the diminutive biopsy. Mod Pathol. 1993. 6: 531-8
8. Davis C, Barnard RO. Malignant behavior of myxopapillary ependymoma. Report of three cases. J Neurosurg. 1985. 62: 925-9
9. Ellison DW, Kocak M, Figarella-Branger D, Felice G, Catherine G, Pietsch T. Histopathological grading of pediatric ependymoma: Reproducibility and clinical relevance in European trial cohorts. J Negat Results Biomed. 2011. 10: 7-
10. Feldman WB, Clark AJ, Safaee M, Ames CP, Parsa AT. Tumor control after surgery for spinal myxopapillary ependymomas: Distinct outcomes in adults versus children. J Neurosurg Spine. 2013. 19: 471-6
11. Ho DM, Hsu CY, Wong TT, Chiang H. A clinicopathologic study of 81 patients with ependymomas and proposal of diagnostic criteria for anaplastic ependymoma. J Neurooncol. 2001. 54: 77-85
12. Kittel K, Gjuric M, Niedobitek G. [Metastasis of a spinal myxopapillary ependymoma to the inner auditory canal]. HNO. 2001. 49: 298-302
13. Liborio R, Pais RF, Soares GB, Rocha A, Ferreira F, Garcia T. [Medullary and intracranial metastases of myxopapillary ependymoma]. Acta Med Port. 2001. 14: 133-8
14. Lim SC, Jang SJ. Myxopapillary ependymoma of the fourth ventricle. Clin Neurol Neurosurg. 2006. 108: 211-4
15. Louis DN OH, Wiestler OD, Cavenee WK.editorsWHO Classification of Tumours of the Central Nervous System. IARC, Lyon Revised; 2016. p.
16. Mavroudis C, Townsend JJ, Wilson CB. A metastasizing ependymoma of the cauda equina. Case report. J Neurosurg. 1977. 47: 771-5
17. McLendon R.E. RMK, Schiffer D, Louis DN, Ohgaki H, Weistler OD.editors. Myxopapillary ependymoma. WHO classification of tumours of the central nervous system. Lyon, France: IARC Press; 2007. p. 72-3
18. Miralbell R, Louis DN, O'Keeffe D, Rosenberg AE, Suit HD. Metastatic ependymoma of the sacrum. Cancer. 1990. 65: 2353-5
19. Pica A, Miller R, Villa S, Kadish SP, Anacak Y, Abusaris H. The results of surgery, with or without radiotherapy, for primary spinal myxopapillary ependymoma: A retrospective study from the rare cancer network. Int J Radiat Oncol Biol Phys. 2009. 74: 1114-20
20. Sonneland PR, Scheithauer BW, Onofrio BM. Myxopapillary ependymoma. A clinicopathologic and immunocytochemical study of 77 cases. Cancer. 1985. 56: 883-93
21. Straus D, Tan LA, Takagi I, O'Toole JE. Disseminated spinal myxopapillary ependymoma in an adult at initial presentation: A case report and review of the literature. Br J Neurosurg. 2014. 28: 691-3
22. Toktas ZO, Demir MK, Yapicier O, Akakin A, Yilmaz B, Konya D. Disseminated adult spinal extramedullary myxopapillary ependymoma. Spine J. 2015. 15: e69-70
23. Weber DC, Wang Y, Miller R, Villa S, Zaucha R, Pica A. Long-term outcome of patients with spinal myxopapillary ependymoma: Treatment results from the MD Anderson Cancer Center and institutions from the Rare Cancer Network. Neuro Oncol. 2015. 17: 588-95
24. Wight DG, Holley KJ, Finbow JA. Metastasizing ependymoma of the cauda equina. J Clin Pathol. 1973. 26: 929-35
25. Woesler B, Moskopp D, Kuchelmeister K, Schul C, Wassmann H. Intracranial metastasis of a spinal myxopapillary ependymoma. A case report. Neurosurg Rev. 1998. 21: 62-5
26. Yucesoy K, Ozer E, Koyuncuoglu M. Parenchymal brain metastasis of a spinal myxopapillary ependymoma after extradural manipulation. Acta Neurochir (Wien). 2001. 143: 1071-2