- Department of Neurosurgery, Kanazawa Medical Center, Kanazawa, Japan
Correspondence Address:
Yu Shimizu
Department of Neurosurgery, Kanazawa Medical Center, Kanazawa, Japan
DOI:10.4103/sni.sni_370_18
Copyright: © 2019 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Yu Shimizu, Katsuhiro Tsuchiya, Hironori Fujisawa. Nocardia paucivorans cerebellar abscess: Surgical and pharmacotherapy. 22-Feb-2019;10:22
How to cite this URL: Yu Shimizu, Katsuhiro Tsuchiya, Hironori Fujisawa. Nocardia paucivorans cerebellar abscess: Surgical and pharmacotherapy. 22-Feb-2019;10:22. Available from: http://surgicalneurologyint.com/surgicalint-articles/9206/
Abstract
Background:Nocardia species are ubiquitous in nature and mainly cause pulmonary disease in humans; however, they can also infect the central nervous system and skin. The management of cerebellar nocardiosis is troublesome and requires multiple considerations of the severity of the underlying systemic disease, difficulties in identifying the bacterium, and frequent delay in initiating adequate therapy.
Case Description:We report a 52-year-old diabetic female patient with Nocardia paucivorans cerebellar abscesses. Brain magnetic resonance imaging (MRI) revealed innumerable small ring-enhancing lesions of posterior fossa. In this report, we present a case of primary single cerebellar abscesses due to N. paucivorans. Early diagnosis and surgical interventions were significant for the patient. The diagnosis was confirmed by DNA sequencing and the organism was susceptible to trimethoprim–sulfamethoxazole (TMP/SMX). The patient was successfully treated with drugs and surgical excision.
Conclusion:According to the literature, surgical excision or aspiration of cerebellar abscess seems to provide favorable outcomes. In our experience, a successful outcome was achieved with subtotal resection and prolonged adequate antibiotic therapy.
Keywords: Cerebellar abscess, brain abscess, Grocott stain, Nocardia infection, Nocardia paucivorans, surgery
INTRODUCTION
Nocardia is a gram-positive, branching, filamentous bacteria, ubiquitous in soil, and is distributed worldwide.[
CASE PRESENTATION
A 52-year-old female patient was admitted to our hospital. The patient presented with ongoing (3 days) ataxia in the right lower limbs, in addition to dizziness and a progressive headache. No fever was noted in the past month before admission. Her medical history was unremarkable; she was immunocompetent, her diabetes was well controlled, and she did not have a history of surgery or steroid abuse. Her social history included intermittent alcohol consumption without smoking. She was alert and responsive on admission. Physical examination revealed clear lung sounds without rales or wheezing. Her heartbeat was regular without any murmurs. There was no tenderness or rebound tenderness in the abdomen. Neurological examination revealed right-sided ataxia and dysarthria. There were no other symptoms, for example, fever, neck stiffness, photophobia, papilledema, or other abnormalities. Laboratory testing revealed a C-reactive protein level of 0.7 mg/dL and a white blood cell count of 8500 μL. The patient had a normal neutrophil function test result, lymphocyte count, and normal immunoglobulin levels. She was tested negative for human immunodeficiency virus (HIV), HIV antibodies, hepatitis B surface antigens, and hepatitis C antibodies. Cerebrospinal fluid revealed a white blood cell count of 18 cells/mm3, 70% lymphocytes (normal: <5% lymphocytes, no neutrophils, no monocytes), a red blood cell count of 1 cells/mm3, and protein level of 92.9 mg/dL (reference range: <45 mg/dL). Cultures were negative for bacterial and fungal infection. A computed tomography (CT) scan of the head showed a right cerebellar low-density lesion without hydrocephalus. A CT scan of the chest, abdomen, and pelvis did not show any abnormalities. Cerebral MRI disclosed multiple necrotic cystic ring-enhancing lesions in the right cerebellar juxtaventricular region with surrounding edema. Diffusion-weighted imaging (DWI) showed restricted diffusion [
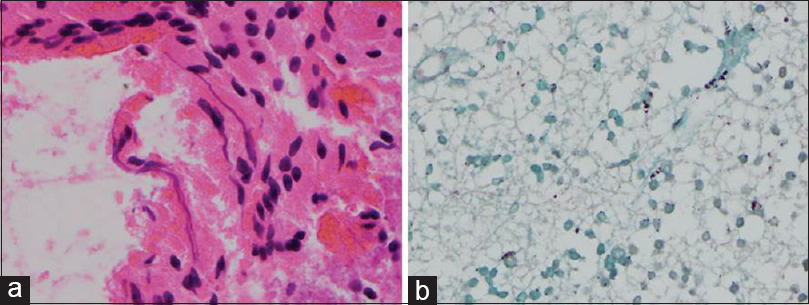
Figure 1
(a) The patient underwent resection of the lesion for microbiological and histopathological examination. Histopathological examination of the brain specimen demonstrated thin, branching organisms of about 1-micron thickness, consistent with Nocardia species on hematoxylin and eosin staining (original magnification, ×40). (b) Grocott staining revealed thin, filamentous, and ramifying argyrophilic bacteria (original magnification, ×40)
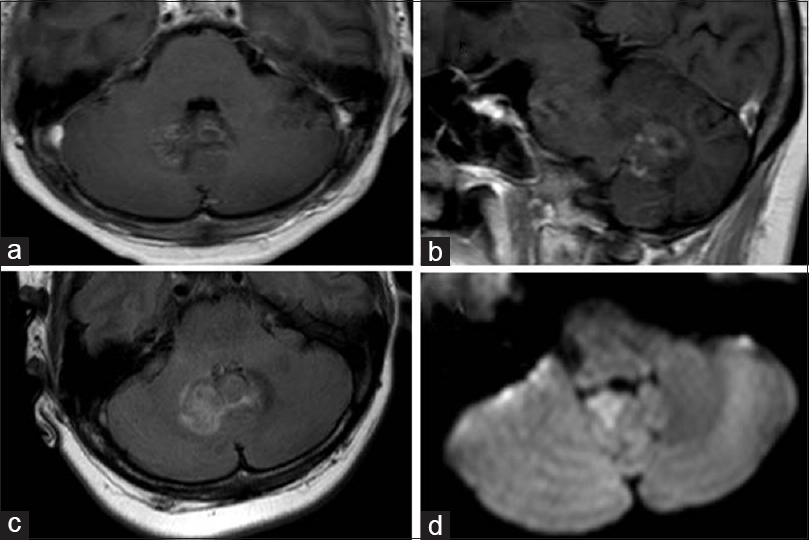
Figure 2
(a and b) T1-enhanced axial, sagittal magnetic resonance image showing infratentorial lesion affecting deep structures, including the cerebellar vermis. The lesion is juxtaventricular (fourth ventricle), but not cause obstructive hydrocephalus. (c) Fluid-attenuated inversion recovery demonstrated brain edema around the lesion. (d) Diffusion-weighted image showing a restricted lesion of abscess
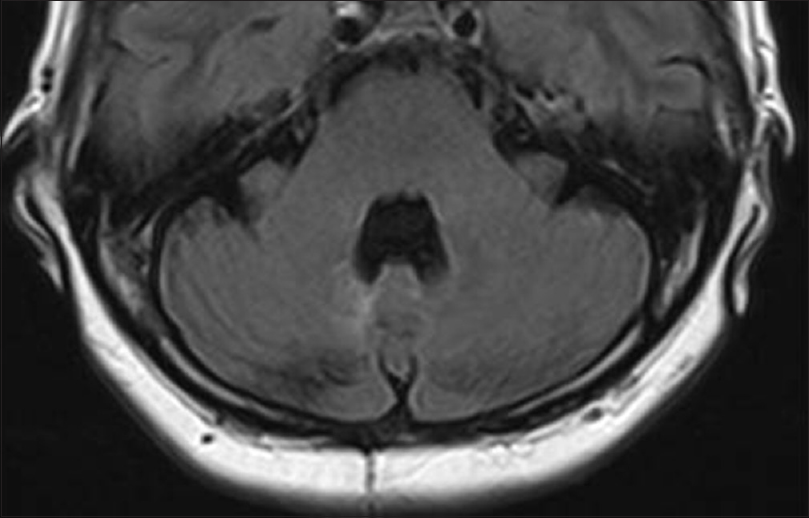
This preliminary in vitro resistance to TMP/SMX informed the choice of ceftriaxone sodium as initial empiric treatment. A subsequent susceptibility E-test confirmed that the pathogen was actually susceptible to TMP/SMX (minimum inhibitory concentration: 0.01 μg/mL); therefore, antimicrobial therapy was modified accordingly. Medical treatment included ceftriaxone sodium (2 g/12 h) for 1 month and TMP-SMX [1600 mg/320 mg intravenous (IV), daily] for 1 month, followed by transition to oral therapy levofloxacin (500 mg daily) and TMP-SMX (1600 mg/320 mg also oral daily) for an additional 11 months. The patient's clinical condition improved over the following 5 weeks, and she was discharged on Day 35 with no neurological deficit. After 1 year of treatment, MRI revealed no brain abscesses [
DISCUSSION
Nocardia accounts for as little as 1–2% of all brain abscesses, and it is a rare cause of brain abscess, particularly in an immunocompetent host.[
Clinical manifestations of brain nocardiosis are commonly insidious and non-specific. Patients are typically diagnosed because of neurologic defects due to mass effect or even incidentally when performing craniotomy for a presumed brain tumor. The mortality rates estimated for a nocardial brain abscess are 55 and 20% in immune-compromised and immune-competent patients, respectively.[
CONCLUSION
Nocardia seems to have a special tropism for the neural tissue. Solitary abscess represents the most common manifestation in the central nervous system, accounting for 1–2% of all cerebral abscesses. Early identification of the specific nocardial species is important to initiate long-term effective antibiotic therapy. In our experience, successful outcome was achieved performing subtotal resection and administering antibiotic therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
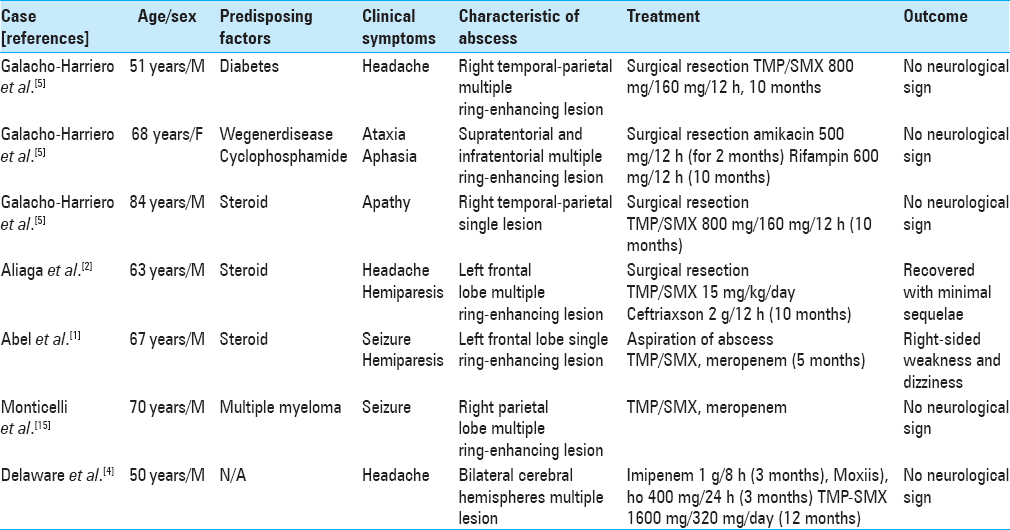
1. Abel S, Hasan S, Kujawski B, Talwar A, Betler J, Wegner R. Cryptic Nocardia nova brain abscess postradiation treatment and neurosurgery in a patient with small cell lung cancer: A case report and review of the literature. Adv Radiat Oncol. 2016. 18: 290-3
2. Aliaga L, Fatoul G, Guirao E, Peña A, Rodríguez-Granger J, Cobo F. Nocardia paucivorans brain abscess. Clinical and microbiological characteristics. ID Cases. 2018. 13: e00422-
3. Chaudhari DM, Renjen PN, Sardana R. Nocardia Farcinica brain abscess in an immunocompetent old patient: A case report and review of literature. Ann Indian Acad Neurol. 2017. 20: 399-402
4. Delaware N, Than KD, Chen KS, McKeever PE, Wang AC, Pandey AS. Resolution of innumerable cerebral Nocardia paucivorans abscesses after medical management. J Clin Neurosci. 2016. 27: 175-7
5. Galacho-Harriero A, Delgado-López PD, Ortega-Lafont MP, Martín-Alonso J, Castilla-Díez JM, Sánchez-Borge B. Nocardia farcinica brain abscess: Report of 3 cases. World Neurosurg. 2017. 106: 1053.e15-1053.e24
6. Haines AB, Zimmerman RD, Morgello S. MR imaging of brain abscesses. AJR Am J Roentgenol. 1989. 152: 1073-85
7. Kim S, Lee KL, Lee DM, Jeong JH. Nocardia brain abscess in an immunocompetent patient. Infect Chemother. 2014. 46: 45-9
8. Kumar VA, Augustine D, Panikar D. Nocardia farcinica brain abscess: Epidemiology, pathophysiology, and literature review. Surg Infect (Larchmt). 2014. 15: 640-6
9. Lai CC, Lee LN, Teng LJ. Disseminated Nocardia farcinica infection in a uraemia patient with idiopathic thrombocytopenia purpura receiving steroid therapy. J Med Microbiol. 2005. 54: 1107-10
10. Laurent FJ, Provost F, Boiron P. Rapid identification of clinically relevant Nocardia species to genus level by 16S rRNA gene PCR. J Clin Microbiol. 1999. 37: 99-102
11. Lee GY, Daniel RT, Brophy BP. Surgical treatment of nocardial brain abscesses. Neurosurgery. 2002. 51: 668-71
12. Lerner PI. Nocardiosis. Clin Infect Dis 199. 2: 891-903
13. Lin YJ, Yang KY, Ho JT. Nocardial brain abscess. J Clin Neurosci. 2010. 17: 250-3
14. Menkü A, Kurtsoy A, Tucer B. Nocardia brain abscess mimicking brain tumour in immunocompetent patients: Report of two cases and review of the literature. Acta Neurochir (Wien). 2004. 146: 411-4
15. Monticelli J, Luzzati R, Maurel C, Rosin C, Valentinotti R, Farina C. Brain abscesses caused by Nocardia paucivorans in a multiple myeloma patient treated with lenalidomide and dexamethasone: A case report and review of literature. Mediterr J Hematol Infect Dis. 2015. 7: e2015011-
16. Rafiei N, Peri AM, Righi E, Harris P, Paterson DL. Central nervous system nocardiosis in Queensland: A report of 20 cases and review of the literature, Medicine (Baltimore). 2016. 95: e5255-
17. Sorrell TC, Mitchell DH, Iredell JR, Chen SC-A, Bennett JE, Dolin R, Blaser MJ.editors. Nocardia species. Philadelphia: Elsevier; 2015. 2: 2853-63