Novel approach to continuous neurophysiological monitoring during surgery of peripheral nerve tumors
- Intraoperative Neurophysiological Monitoring Unit, Hospital Universitario de Canarias, San Cristobal de la Laguna, Santa Cruz de Tenerife, Spain
Correspondence Address:
Ángel Saponaro-González
Intraoperative Neurophysiological Monitoring Unit, Hospital Universitario de Canarias, San Cristobal de la Laguna, Santa Cruz de Tenerife, Spain
DOI:10.4103/sni.sni_414_16
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ángel Saponaro-González, Pedro Javier Pérez-Lorensu. Novel approach to continuous neurophysiological monitoring during surgery of peripheral nerve tumors. 10-Aug-2017;8:184
How to cite this URL: Ángel Saponaro-González, Pedro Javier Pérez-Lorensu. Novel approach to continuous neurophysiological monitoring during surgery of peripheral nerve tumors. 10-Aug-2017;8:184. Available from: http://surgicalneurologyint.com/surgicalint-articles/novel-approach-to-continuous-neurophysiological-monitoring-during-surgery-of-peripheral-nerve-tumors/
Abstract
Background:Intraoperative neurophysiological monitoring (IONM) with nerve action potential (NAP) can be useful during peripheral nerve surgery. However, current methodologies are not optimized for continuous recording of the NAP. The use of newer electrodes may make it possible to more conveniently obtain continuous recordings of the NAP during surgery.
Methods:After localizing the nerve of interest and dissecting it from the adjacent soft tissue, two APS® (Automatic Periodic Stimulation) electrodes, originally designed for stimulation of the vagus nerve during thyroid surgery, are placed on the nerve on either sides of the tumor for stimulation and recording using two subdermal electroencephalogram (EEG) needles as anode and reference, respectively. Both monopolar and bipolar recordings can be used as appropriate. Anesthesia regime comprised sevoflurane or total intravenous anesthesia (TIVA). No muscle relaxant after intubation, local anesthesia, or blood pressure cuff is used during the surgery.
Results:Twelve patients (6 male, 6 female) with peripheral nerve tumors (motor, sensitive, or mixed nerves) or tumors affecting the peripheral nerves were monitored in our center since 2014 (mean age: 50 years; 28–79). In 10 patients, the NAP was monitored without experiencing any changes from the beginning till the end of the surgery; in these patients, no postoperative deficit was adverted. In the last 2 patients, who departed from a complete neurological deficit, no NAP was recorded at the baseline or during the surgery, and they did not experience any neurological improvement.
Conclusion:The vagus nerve stimulation electrodes open new possibilities in peripheral nerve IONM. We have used them for continuous monitoring without additional problems with the traditional probes.
Keywords: Nerve tumor, neurophysiological intraoperative monitoring, peripheral nerve
INTRODUCTION
During the last two decades, intraoperative neuromonitoring (IONM) has experienced a huge development in the fields of spine, posterior fossa, and brain surgery. Despite this, peripheral nerve surgery has been less covered.
The intraoperative nerve action potential (NAP) is useful in assessing the functional integrity of the nerve when required during peripheral nerve surgery. NAP is an electrical potential recorded from an exposed nerve in response to its electrical stimulation.
In the literature, the most important group who laid the groundwork was the Louisiana State University Medical Center;[
Peripheral nerve surgery comprises acute neurological lesions, neuromas, and peripheral nerve tumors. Although peripheral nerve tumors can be of several types, schwannoma, a tumor developed from the Schwann cells, is the most common. Advanced anatomical knowledge and extensive experience in microsurgery are required to recover the maximum functional capacity and to eliminate pain. Acute lesions and neuromas are treated through direct nerve repair or through a graft whereas tumoral lesions must be resected. In any case, the main goal of peripheral nerve surgery is the preservation of a maximum of undamaged nerve fascicles.
After the work of the Louisiana group, commercial probes for stimulation and recording are widely available. To our advice, theses probes are useful while localizing, identifying, and assessing the functional integrity of the nerve of interest, however, they do not allow continuous monitoring during peripheral nerve tumor removal. To obtain NAP with these probes, the surgeon should elevate the nerve respect to the surgical field to avoid current dispersion. So far, the described technique has been used only for mapping purposes.
MATERIALS AND METHODS
Informed consent and approval from the ethics committee of our institution were obtained for this technical note. The authors declare no conflict of interest.
Twelve patients (6 males and 6 females) have been monitored with this technique in our center since 2014 (Mean age: 50 years; 28–79). Eleven patients were affected by a schwannoma (on 3 median, 2 spinal accessory, 2 sural, 1 superficial peroneal, and 1 peroneal nerve), 2 patients were affected by a neuroma (on a median and a radial nerve), and 1 patient with a peroneal nerve was affected by a sarcoma of the knee. All patients had mild motor impairment or paresthesia, except the patient affected by a sarcoma and the one having a posttraumatic radial neuroma after a humeral fracture who had an almost complete deficit. Anesthesia regime comprised sevoflurane or total intravenous anesthesia (TIVA). No muscle relaxant after intubation, peripheral nerve/plexus blocks for anesthesia, or blood pressure cuff was used during the surgery.
After localizing the nerve of interest, dissecting it from the adjacent soft tissue, and assessing its anatomical integrity, depending on the length of nerve exposed, monopolar or bipolar stimulation is chosen. If there is not enough nerve exposed (<6 cm), monopolar stimulation is performed placing two APS® (Automatic Periodic Stimulation, Medtronic) electrodes on the nerve on either sides of the tumor using subdermal electroencephalogram (EEG) needles as anode for stimulation and as reference (G2) for recording. If enough nerve is exposed (>6 cm), bipolar stimulation placing paired APS® electrodes on either side of the tumor is feasible [
Figure 1
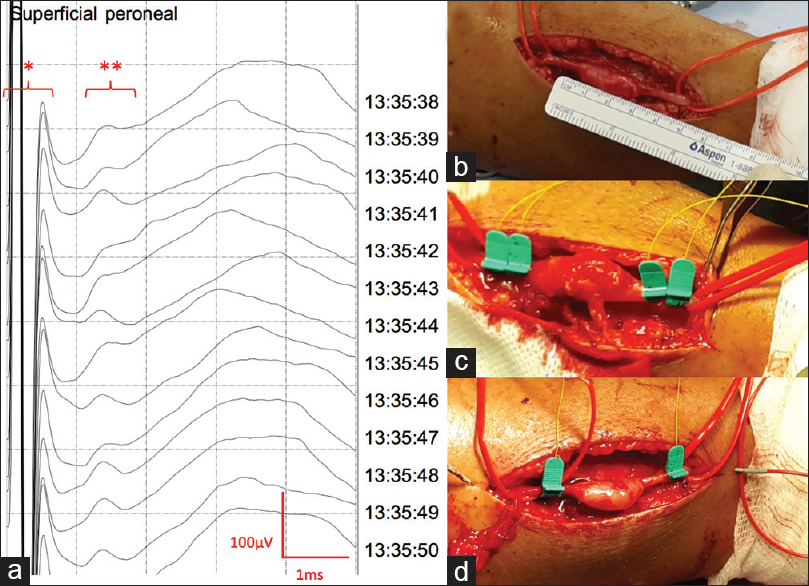
(a) Nerve action potential (NAP) recorded on a superficial peroneal nerve affected by a schwannoma with APS® electrodes following monopolar stimulation and recording. * Stimulus artifact, ** NAP, scale 1 ms/Div; 100 μV/Div. (b) Exposed superficial peroneal nerve showing a schwannoma measuring 3 cm. (c) Bipolar stimulation and recording positioning (this modality was dismissed since it does not leave enough exposed nerve to work). (d) Monopolar stimulation and recording positioning (this modality was used to record the NAP showed in a)
Even though we usually perform monopolar stimulation, the distance between the poles for stimulation and recording ranges 5 to 9 mm. We have not experienced an increase in stimulus artifact, which could be in part due to the characteristics of the electrode, having a tiny active surface surrounded by plastic that isolates the nerve from current dispersion.
To obtain NAP some technical aspects should be taken into account; regarding stimulation parameters, distance between stimulation and recording points should be over 4 cm, the stimulus duration should be short (0.05–0.1 ms), with square pulse, intensity to evoke a supramaximal potential generally does not need to be more than 1–5 mA. Regarding recording parameters, low pass filter should be 5–10 Hz and high pass filter ranging 2000–3000 Hz, sensitivity should be set at 20–50 μV/cm, and epoch length should be set at 10 ms.[
RESULTS
Among 10 patients undergoing a peripheral nerve surgery (schwannoma or neuroma), the nerve was localized and monitored without experiencing changes in the NAP amplitude. The patients did not have any preoperative or postoperative deficit, and hence, they were considered as true negatives. In the other 2 patients (a peroneal nerve affected by a sarcoma of the knee and a radial neuroma developed after a humeral fracture fixed with a plate), no NAP or compound muscle action potential (CMAP) was recorded at the baseline. These patients had a severe preoperative deficit and there were no clinical changes at outcome; hence, they were considered true positives.
DISCUSSION
The main advantages of this technique are that it allows continuous monitoring of NAP without further intervention of the surgeon or the assistant, it offers more visibility inside the surgical field during the removal of a peripheral nerve tumor, and there are no additional technical issues compared with the traditional materials used for monitoring peripheral nerves.
On the other hand, the main limitations are that there are only two sizes are commercially available for the APS® electrode: 2.0 and 3.0 mm models for nerves ranging 2–3 and 3–4 mm, respectively, and that if the diameter of the nerve of interest is smaller or bigger than the diameter of the electrode it could migrate or cause a nerve local damage. In the mentioned situation, this methodology is not appropriate and traditional probes are used. Due to the limited number of patients in our series, no intraoperative changes regarding the amplitude of the NAP were recorded; therefore, it is not possible to establish a correlation between the intraoperative findings and the outcome.
CONCLUSION
To our knowledge, this is a feasible and reliable methodology that allows continuous monitoring of the functional integrity of peripheral nerve during peripheral nerve tumor surgery. The vagus nerve stimulation electrodes, initially designed for thyroid surgery, open a new range of possibilities in the peripheral nerve neurophysiological monitoring. Longer case series are needed to establish the correlation between intraoperative neurophysiological changes and clinical outcome.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Happel L, Kline D, Deletis V, Shils J.editors. Intraoperative neurophysiology of the peripheral nervous system. Neurophysiology in neurosurgery: A modern intraoperative approach. New York: Elsevier; 2002. p.
2. Herrera-Pérez M, Oller-Boix A, Pérez-Lorensu PJ, de Bergua-Domingo J, Gonzalez-Casamayor S, Márquez-Marfil F. Intraoperative neurophysiological monitoring in peripheral nerve surgery: Technical description and experience in a center. Rev Esp Cir Ortop Traumatol. 2015. 59: 266-74
3. Robert EG, Happel LT, Kline DG. Intraoperative nerve action potential recordings: Technical considerations, problems, and pitfalls. Neurosurgery. 2009. 65: A97-104