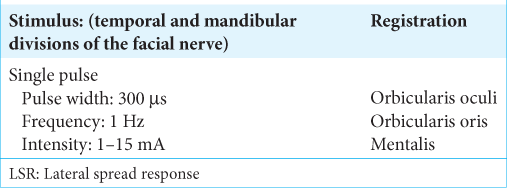
- Department of Neuroscience, Neurosurgery Section, Università Cattolica del Sacro Cuore, Rome, Italy
- Department of Neurosurgery, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy
- Neurosurgical Intensive Care Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy
Correspondence Address:
Nicola Montano, Department of Neuroscience, Neurosurgery Section, Università Cattolica del Sacro Cuore, Rome, Italy
DOI:10.25259/SNI_268_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Marco Battistelli1, Alessandro Izzo2, Manuela D’Ercole2, Quintino Giorgio D’Alessandris1, Michele Di Domenico2, Eleonora Ioannoni3, Camilla Gelormini3, Renata Martinelli1, Federico Valeri1, Fulvio Grilli1, Nicola Montano1. Optimizing surgical technique in microvascular decompression for hemifacial spasm – Results from a surgical series with contemporary use of neuronavigation and intraoperative neuromonitoring. 06-Sep-2024;15:319
How to cite this URL: Marco Battistelli1, Alessandro Izzo2, Manuela D’Ercole2, Quintino Giorgio D’Alessandris1, Michele Di Domenico2, Eleonora Ioannoni3, Camilla Gelormini3, Renata Martinelli1, Federico Valeri1, Fulvio Grilli1, Nicola Montano1. Optimizing surgical technique in microvascular decompression for hemifacial spasm – Results from a surgical series with contemporary use of neuronavigation and intraoperative neuromonitoring. 06-Sep-2024;15:319. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13083
Abstract
Background: Microvascular decompression (MVD) through a retrosigmoid approach is considered the treatment of choice in cases of hemifacial spasm (HFS) due to neurovascular conflict (NVC). Despite the widespread of neuronavigation and intraoperative neuromonitoring (IONM) techniques in neurosurgery, their contemporary application in MVD for HFS has been only anecdotally reported.
Methods: Here, we report the results of MVD performed with a combination of neuronavigation and IONM, including lateral spread response (LSR) in 20 HFS patients. HFS clinical outcome and different surgical-related factors, such as craniotomy size, surgical duration, mastoid air cell (MAC) opening, postoperative cerebral spinal fluid (CSF) leakage, sinus injury, and other complications occurrence, and the length of hospitalization (LOS) were studied.
Results: Postoperatively, residual spasm persisted only in two patients, but at the latest follow-up (FU) (mean: 12.5 ± 8.98 months), all patients had resolution of symptoms. The mean surgical duration was 103.35 ± 19.36 min, and the mean LOS was 2.21 ± 1.12 days. Craniotomy resulted in 4.21 ± 1.21 cm2 in size. Opening of MAC happened in two cases, whereas no cases of CSF leak were reported as well as no other complications postoperatively and during FU.
Conclusion: MVD for HFS is an elective procedure, and for this reason, surgery should integrate all technologies to ensure safety and efficacy. The disappearance of LSR is a crucial factor for identifying the vessel responsible for NVC and for achieving long-term resolution of HFS symptoms. Simultaneously, the benefits of using neuronavigation, including the ability to customize the craniotomy, contribute to reduce the possibility of complications.
Keywords: Hemifacial spasm, Intraoperative neuromonitoring, Lateral spread response, Microvascular decompression, Neuronavigation
INTRODUCTION
Hemifacial spasm (HFS) is a neurological disorder characterized by recurrent, involuntary twitching of facial muscles located on one side of the face, receiving innervation from the ipsilateral facial nerve. Classified within the peripheral neuromuscular movement disorder category, the spasmodic contractions typically originate in the orbicularis oculi muscle and gradually extend to encompass other muscles innervated by the facial nerve on that side. Etiologically, HFS is classified into (1) a primary form, linked to neurovascular conflict (NVC); (2) hereditary, marked by a robust family history of HFS in first-degree relatives; (3) secondary to an identifiable cause (such as Bell’s palsy, facial nerve injury, demyelinating pathologies, or vascular insults); and (4) HFS mimickers, encompassing psychogenic, tics, dystonia, myoclonus, myokymia, myorhythmia, and hemimasticatory spasm.[
MATERIALS AND METHODS
Patients
The present series includes 20 consecutive patients suffering from medically intractable HFS who underwent MVD at our institution between January 2022 and July 2023. The inclusion criteria were the following: (1) confirmed clinical and neurophysiological diagnosis of HFS and (2) a preoperative brain magnetic resonance imaging (MRI) comprising time-of-flight and fast imaging employing steady-state acquisition sequences showing NVC at the facial REZ. The exclusion criteria were the following: (1) MRI showing structural lesions such as tumors, arteriovenous malformation, and Chiari I malformation and (2) patients with blepharospasm, facial myokymia, or Meige syndrome.
All patients were operated by the senior author (NM). All patients gave informed consent both to the surgical procedure and to the present study. Ethics committee review/approval was not needed due to the retrospective nature of this study.
Surgical procedure
All procedures were performed under general anesthesia in a park bench position that, in our experience, provides easy and comfortable access to the cerebellopontine angle.[
IONM
NIM eclipse system (Medtronic) was used for IONM. DES of facial cranial nerves was used to identify the neural structures correctly. A monopolar constant-current low-frequency stimulation was used for DES in all cases (monopolar probe [cathodal] 300 μs, 1 Hz, 0.1–0.5 mA). Usually, an intensity of stimulus at 0.1 mA, delivered on the nerve surface, is enough to record a compound muscle action potential. The need for higher stimulus intensity might indicate a relative distance to the neural structure or the presence of a relevant tissue barrier between the probe and the nerve. The more motor response is evoked with low intensity, the more the surgeon is working close to the nerve. At the end of the surgery, we always perform a proximal stimulation at a low threshold (0.1–0.3 mA) to confirm the functional facial nerve integrity. Entire cranial nerves motor pathways integrity was evaluated using corticobulbar motor evoked potentials.[
Outcome indicators
The following parameters were recorded: craniotomy size, surgical duration, mastoid air cell (MAC) opening, postoperative CSF leakage, sinus injury, and other complications occurrence, length of hospitalization (LOS), and HFS outcome at hospital admission at discharge and latest follow-up (FU) according to Samsung Medical Center (SMC) grading system for severity of HFS spasms [Lee’s scale;
RESULTS
Demographic data
Demographic data are listed in
Outcome data
The outcome results are reported in
Cases illustration
Case 1
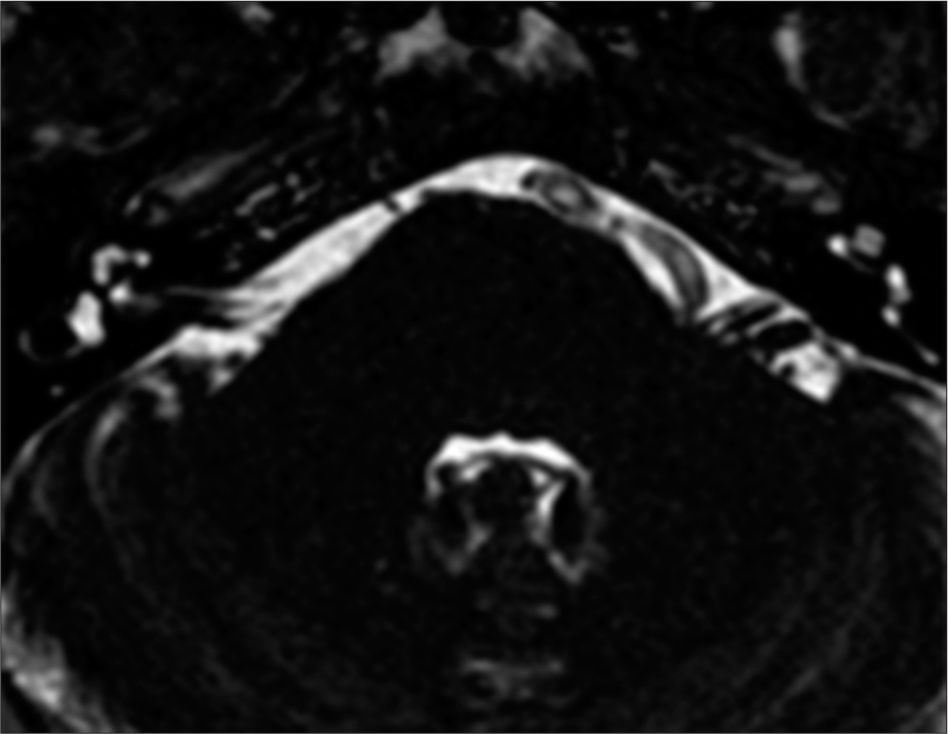
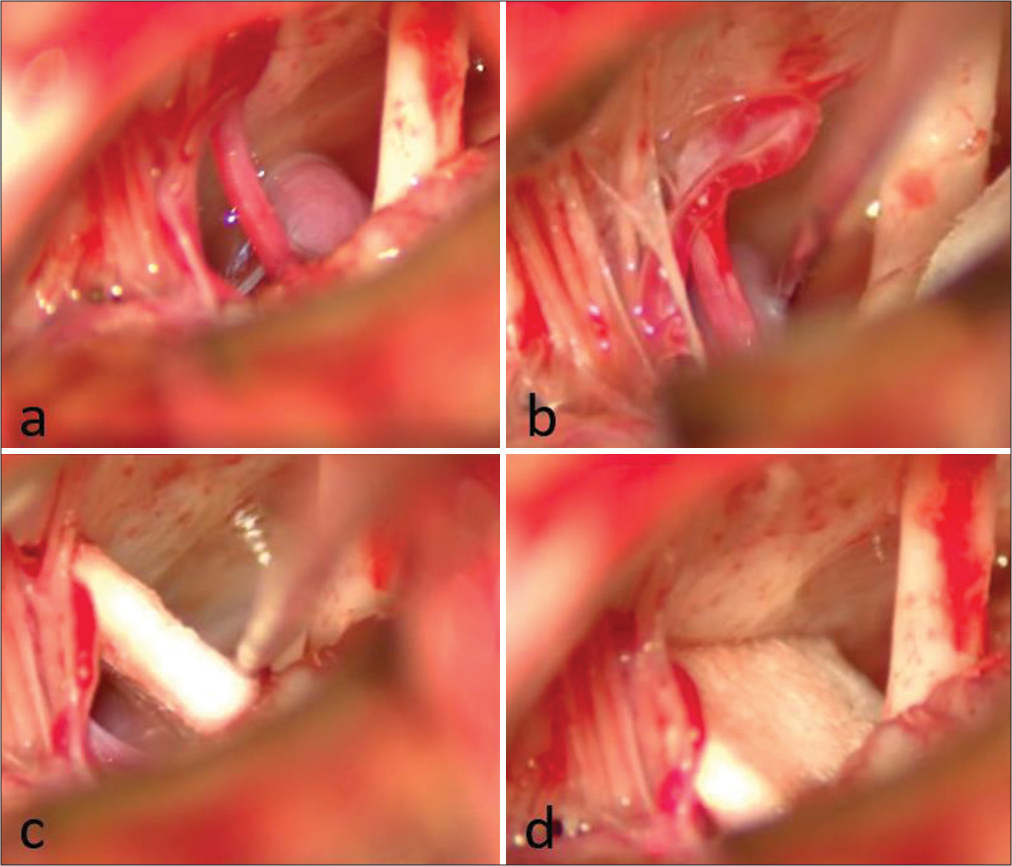
A 59-year-old male patient presented at our institution’s outpatient clinic with a 3-year history of left HFS. The symptoms initially manifested as spasms in the left orbicularis oculi muscle, gradually progressing to involve the remaining left facial muscles, excluding the platysma. In addition, the frequency of spasm episodes increased to 10 per day. Electromyography revealed spontaneous sporadic bursts in the left orbicularis oculi. Brain MRI identified a dolichoectatic left VA compressing the left seventh nerve at its REZ [
Figure 3:
Intraoperative findings of Case 1. (a) Neurovascular conflict between the left vertebral artery (VA) and the left seventh nerve. (b) Nerve hook is utilized for dissecting the VA from the nerve. (c) Following Teflon positioning, the nerve hook is employed to push the VA away from the nerve. (d) Final result after Teflon release.
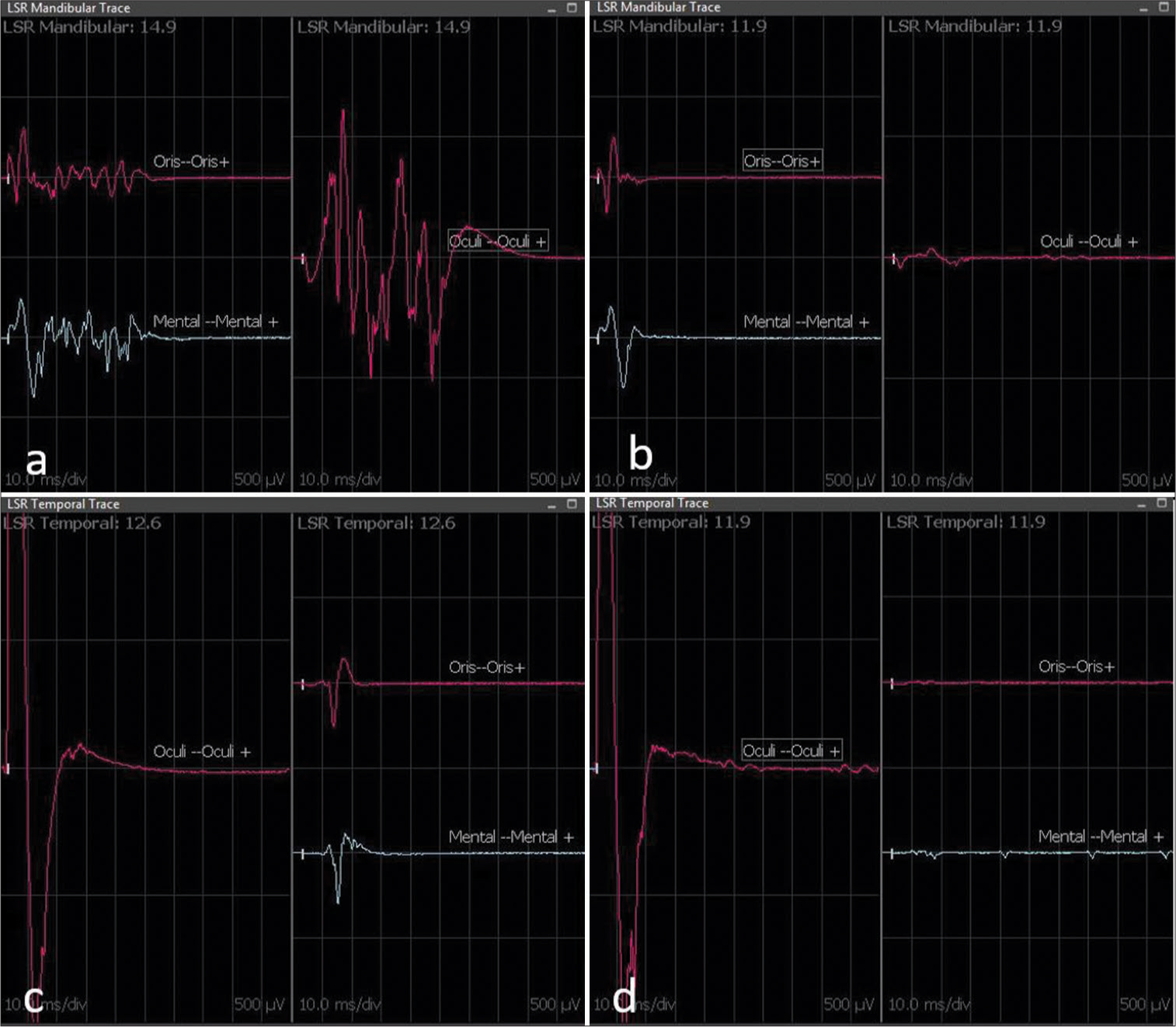
Figure 4:
Lateral spread response (LSR) intraoperative recording of Case 1. The upper figure (a and b) displays the LSR mandibular recording, while the lower one (c and d) shows the LSR temporal recording. LSR records on the left (a and c) are before decompression and Teflon placement, while those on the right (b and d) are after decompression and Teflon placement. Immediately after Teflon placement between the vessel culprit and the seventh nerve, the disappearance of pathological LSR is evident (b and d). (b) Disappearance of LSR at the orbicularis oculi. (d) Disappearance of LSR at the orbicularis oris and mentalis muscle.
Figure 5:
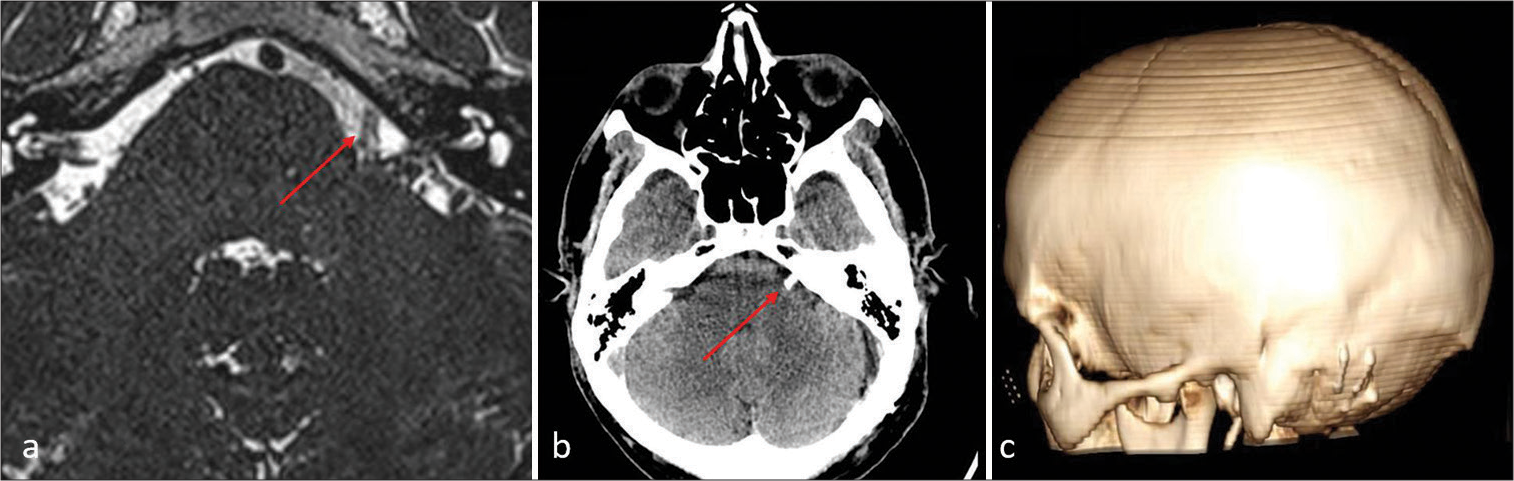
Postoperative fast imaging employing steady-state acquisition magnetic resonance imaging (a) and computed tomography-scan (b and c) of Case 1. (a and b) The Teflon (red arrow) is evident, separating the vertebral artery and facial nerve root entry zone. (c) The extent of the craniotomy and reconstruction is depicted in 3D reconstruction.
Case 2
A 54-year-old male patient was admitted to our department with a 10-year history of right HFS, initially involving the right orbicularis oculi and subsequently extending to the remaining right face, excluding the platysma. A brain MRI revealed a NVC at the REZ of the right facial nerve with the ipsilateral AICA [
Figure 7:
Intraoperative view of Case 2. (a) Neurovascular conflict at the root entry zone of the right facial nerve with the ipsilateral anterior inferior cerebellar artery before and (b) after decompression and Teflon placement. A persistence of lateral spread response was noted, and further exploration was needed.
Figure 8:
Lateral spread response (LSR) recording of Case 2. (a) The figure shows the LSR of the temporal branch. The normal response is one recorded in the orbicularis oculi muscle, (b) while the pathological response is one recorded in the orbicularis oris and (c) mentalis muscles. Immediately after Teflon placement between the anterior inferior cerebellar artery and the right facial nerve (11:16 on the timeline, white triangle), the persistence of pathological LSR in the orbicularis oris (b) and mentalis (c) muscles was noted. Thus, further exploration of the right seventh cranial nerve was continued identifying a second neurovascular conflict between the right facial nerve and the labyrinthine artery. Only after decompression and Teflon placement between the labyrinthine artery and right facial nerve (11:36 on the timeline, yellow triangle) the disappearance of pathological LSR on the orbicularis oris (b) and mentalis (c) muscles was evident. The normal response in the orbicularis oculi muscle (a) is always retained.
DISCUSSION
HFS significantly affects patients’ quality of life, causing disruptions in sleep, social embarrassment, and potential vision impairment in the affected eye.[
In the context of HFS management with MVD, IONM, which includes LSR, plays a significant role. LSR is defined as a pathological latent, abnormal response elicited by the stimulation of one branch of the facial nerve in patients with HFS, resulting in the contraction of the facial muscles innervated by the other branch of the facial nerve. This phenomenon likely arises from the cross-transmission of antidromic activity from the stimulated branch of the facial nerve. A retrospective analysis by Song et al. on 73 patients who underwent MVD for HFS demonstrated significantly higher short-term and long-term spasm resolution in the group where LSR disappeared compared to the group where LSR persisted.[
To the best of our knowledge, this is the largest series focusing on neuronavigated MVD for HFS. The only previously published series on this topic included only 12 HFS patients.[
CONCLUSION
In conclusion, the contemporary use of neuronavigation and IONM with LSR in MVD for HFS seems to enhance the safety/efficacy balance of this procedure because the disappearance of LSR helps in identifying the vessel responsible for NVC to achieve long-term resolution of HFS symptoms. Simultaneously, the benefits of using neuronavigation, including the ability to customize the craniotomy, contribute to reducing the possibility of complications.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Da Silva EB, Leal AG, Milano JB, da Silva LF, Clemente RS, Ramina R. Image-guided surgical planning using anatomical landmarks in the retrosigmoid approach. Acta Neurochir (Wien). 2010. 152: 905-10
2. Della Pepa GM, Montano N, Lucantoni C, Alexandre AM, Papacci F, Meglio M. Craniotomy repair with the retrosigmoid approach: The impact on quality of life of meticulous reconstruction of anatomical layers. Acta Neurochir (Wien). 2011. 153: 2255-8
3. Della Pepa GM, Stifano V, D’Alessandris QG, Menna G, Burattini B, Di Domenico M. Intraoperative corticobulbar motor evoked potential in cerebellopontine angle surgery: A clinically meaningful tool to predict early and late facial nerve recovery. Neurosurgery. 2022. 91: 406-13
4. Di Carlo DT, Benedetto N, Perrini P. Clinical outcome after microvascular decompression for trigeminal neuralgia: A systematic review and meta-analysis. Neurosurg Rev. 2022. 46: 8
5. El Damaty A, Rosenstengel C, Matthes M, Baldauf J, Schroeder HW. The value of lateral spread response monitoring in predicting the clinical outcome after microvascular decompression in hemifacial spasm: A prospective study on 100 patients. Neurosurg Rev. 2016. 39: 455-66
6. Fernández-Conejero I, Ulkatan S, Sen C, Deletis V. Intra-operative neurophysiology during microvascular decompression for hemifacial spasm. Clin Neurophysiol. 2012. 123: 78-83
7. Gardner WJ. Concerning the mechanism of trigeminal neuralgia and hemifacial spasm. J Neurosurg. 1962. 19: 947-58
8. Guan HX, Zhu J, Zhong J. Correlation between idiopathic hemifacial spasm and the MRI characteristics of the vertebral artery. J Clin Neurosci. 2011. 18: 528-30
9. Hatem J, Sindou M, Vial C. Intraoperative monitoring of facial EMG responses during microvascular decompression for hemifacial spasm. Prognostic value for long-term outcome: A study in a 33-patient series. Br J Neurosurg. 2001. 15: 496-9
10. Helal A, Graffeo CS, Meyer FB, Pollock BE, Link MJ. Predicting long-term outcomes after microvascular decompression for hemifacial spasm according to lateral spread response and immediate postoperative outcomes: A cohort study. J Neurosurg. 2024. 140: 1664-71
11. Izzo A, Stifano V, Della Pepa GM, Di Domenico M, D’Alessandris QG, Menna G. Tailored approach and multimodal intraoperative neuromonitoring in cerebellopontine angle surgery. Brain Sci. 2022. 12: 1167
12. Jian ZH, Sheng MF, Li JY, An DZ, Weng ZJ, Chen G. Developing a method to precisely locate the keypoint during craniotomy using the Retrosigmoid Keyhole approach: Surgical anatomy and technical nuances. Front Surg. 2021. 8: 700777
13. Kaufmann AM, Price AV. A history of the Jannetta procedure. J Neurosurg. 2019. 132: 639-46
14. Lee JA, Jo KW, Kong DS, Park K. Using the new clinical grading scale for quantification of the severity of hemifacial spasm: Correlations with a quality of life scale. Stereotact Funct Neurosurg. 2012. 90: 16-9
15. Legninda Sop FY, D’Ercole M, Izzo A, Rapisarda A, Ioannoni E, Caricato A. The impact of neuronavigation on the surgical outcome of microvascular decompression for trigeminal neuralgia. World Neurosurg. 2021. 149: 80-5
16. Lu AY, Yeung JT, Gerrard JL, Michaelides EM, Sekula RF, Bulsara KR. Hemifacial spasm and neurovascular compression. ScientificWorldJournal. 2014. 2014: 349319
17. Mazlout H, Kamoun Gargouri H, Triki W, Kéfi S, Brour J, El Afrit MA. Safety and efficacy of botulinum toxin in hemifacial spasm. J Fr Ophtalmol. 2013. 36: 242-6
18. Menna G, Battistelli M, Rapisarda A, Izzo A, D’Ercole M, Olivi A. Factors related to hemifacial spasm recurrence in patients undergoing microvascular decompression-a systematic review and meta-analysis. Brain Sci. 2022. 12: 583
19. Moffat DA, Durvasula VS, Stevens King A, De R, Hardy DG. Outcome following retrosigmoid microvascular decompression of the facial nerve for hemifacial spasm. J Laryngol Otol. 2005. 119: 779-83
20. Møller AR, Jannetta PJ. Microvascular decompression in hemifacial spasm: Intraoperative electrophysiological observations. Neurosurgery. 1985. 16: 612-8
21. Montano N, Giordano M, Caccavella VM, Ioannoni E, Polli FM, Papacci F. Hemopatch® with fibrin glue as a dural sealant in cranial and spinal surgery. A technical note with a review of the literature. J Clin Neurosci. 2020. 79: 144-7
22. Orlev A, Jackson CM, Luksik A, Garzon-Muvdi T, Yang W, Chien W. Natural history of untreated transverse/sigmoid sinus thrombosis following posterior fossa surgery: Case series and literature review. Oper Neurosurg (Hagerstown). 2020. 19: 109-16
23. Rapisarda A, Orlando V, Izzo A, D’Ercole M, Polli FM, Visocchi M. New tools and techniques to prevent CSF leak in cranial and spinal surgery. Surg Technol Int. 2022. 40: 399-403
24. Raso JL, Gusmão SN. A new landmark for finding the sigmoid sinus in suboccipital craniotomies. Neurosurgery. 2011. 68: 1-6
25. Sharma R, Garg K, Agarwal S, Agarwal D, Chandra PS, Kale SS. Microvascular decompression for hemifacial spasm: A systematic review of vascular pathology, long term treatment efficacy and safety. Neurol India. 2017. 65: 493-505
26. Song H, Xu S, Fan X, Yu M, Feng J, Sun L. Prognostic value of lateral spread response during microvascular decompression for hemifacial spasm. J Int Med Res. 2019. 47: 6120-8
27. Wang J, Zhang W, Wang X, Luo T, Wang X, Qu Y. Application of neuronavigation in microvascular decompression: Optimizing craniotomy and 3D reconstruction of neurovascular compression. J Craniofac Surg. 2023. 34: e620-3
28. Yaltho TC, Jankovic J. The many faces of hemifacial spasm: Differential diagnosis of unilateral facial spasms. Mov Disord. 2011. 26: 1582-92
29. Yanagawa T, Hatayama T, Harada Y, Sato E, Yamashita K, Tanaka M. Preoperative risk assessment for predicting the opening of mastoid air cells in lateral suboccipital craniotomy for microvascular decompression. Clin Neurol Neurosurg. 2020. 189: 105624
30. Ying TT, Li ST, Zhong J, Li XY, Wang XH, Zhu J. The value of abnormal muscle response monitoring during microvascular decompression surgery for hemifacial spasm. Int J Surg. 2011. 9: 347-51
31. Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006. 31: 1116-28
32. Zagui RM, Matayoshi S, Moura FC. Adverse effects associated with facial application of botulinum toxin: A systematic review with meta-analysis. Arq Bras Oftalmol. 2008. 71: 894-901
33. Zhang KW, Shun ZT. Microvascular decompression by the retrosigmoid approach for idiopathic hemifacial spasm: Experience with 300 cases. Ann Otol Rhinol Laryngol. 1995. 104: 610-2