- Department of Neurosurgery, Mohammed V University of Rabat, Faculty of Medicine and Pharmacy of Rabat - Morocco, Rabat, Morocco
Correspondence Address:
Yao Christian Hugues Dokponou, Department of Neurosurgery, Mohammed V University of Rabat, Faculty of Medicine and Pharmacy of Rabat - Morocco, Rabat, Morocco.
DOI:10.25259/SNI_98_2025
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mohammed Yassaad Oudrhiri, Hajar Bechri, Yao Christian Hugues Dokponou, Yasser Arkha, Abdessamad El Ouahabi. Patient selection criteria and preliminary outcome of the first 20 endoscopic evacuation of intracerebral hematoma in a tertiary hospital center. 23-May-2025;16:190
How to cite this URL: Mohammed Yassaad Oudrhiri, Hajar Bechri, Yao Christian Hugues Dokponou, Yasser Arkha, Abdessamad El Ouahabi. Patient selection criteria and preliminary outcome of the first 20 endoscopic evacuation of intracerebral hematoma in a tertiary hospital center. 23-May-2025;16:190. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13579
Abstract
Background: Evacuation of intracerebral hemorrhage (ICH) using endoscopic, minimally invasive surgery is becoming the main technique in the surgical treatment of this devastating disease, given the overall improved outcomes reported. We report our experience with patient selection and preliminary results of the first 20 patients with ICH treated with endoscopic evacuation.
Methods: A retrospective analysis of intraparenchymal and/or intraventricular hemorrhage cases, treated from 2018 to 2020 was performed. Patient characteristics, technical details, and surgical outcomes (favorable, modified Rankin scale [mRS] 0–2; unfavorable, mRS 3–5; death, and mRS 6) were analyzed and discussed.
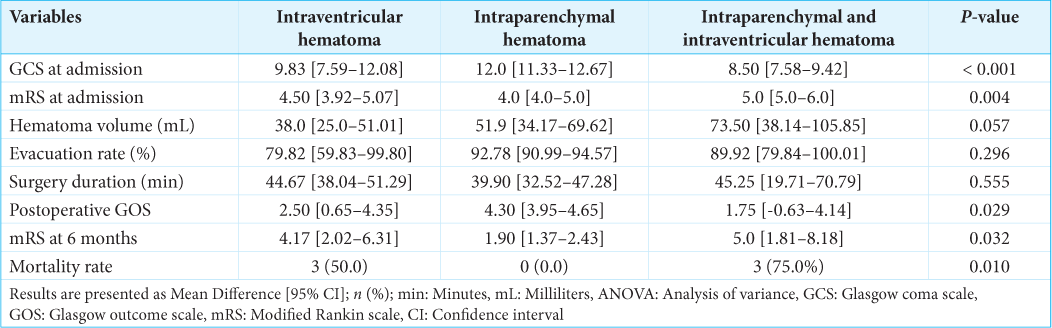
Results: Six (30.0%) cases of IVH, 10 (50.0%) of intraparenchymal hematoma (IP), and 4 (20.0%) of IP&IVH were treated using the endoscopic technique. The mean age was 50.8 [17.6] years, with a male predominance of 60.0% (n = 12). Analysis of variance testing of the mean difference confirmed a favorable outcome when the hemorrhage was limited to the IP location (mean mRS score at 6 months was 1.90 (95% confidence interval [CI] [1.37–2.43], P = 0.032). However, there was an unfavorable outcome when blood was inside the ventricles: IVH (mean mRS at 6 months was 4.17 (95% CI [2.02–6.31], P = 0.032) and IP&IVH (mean mRS at 6 months was 5.0 (95% CI [1.81–8.18], P = 0.032).
Conclusion: The endoscopic intracranial hematoma evacuation technique can achieve a high evacuation rate with shorter surgical duration and acceptable morbidity, encouraging the transition from classical craniotomy in selected patients. Sufficient knowledge and training in endoscopic techniques can be achieved through a short learning curve.
Keywords: Endoscopic, Intracranial hematoma, Minimally invasive, Outcome, Patient selection
INTRODUCTION
Surgical removal of intracerebral hemorrhage (ICH) aims to reduce the mass effect, control intracranial pressure, and prevent or alleviate hernias and the neurotoxicity of blood degradation products.[
Moreover, Pradilla et al.[
However, previous comparative pilot studies failed to demonstrate the superiority of surgical management over medical treatment. The American Heart Association (AHA) guidelines consider surgical indications in two main situations: worsening lobar hematomas measuring 1 cm above the cortical surface and cerebellar hematomas >15 mL in volume; however, its benefits are otherwise not well established or uncertain.[
One explanation offered by the majority of surgeons for the dearth of scientific data regarding the evacuation of intracerebral hematomas is surgical trauma: cortical aggression and manipulation, duration of the surgical procedure, and blood loss are all thought to have a detrimental effect on the postoperative course in patients who are already fragile.
Therefore, endoscopic hematoma evacuation has been considered a promising technique in recent decades, with several case series and meta-analyses assessing its potential superiority over classical craniotomy in terms of invasiveness, evacuation rates, morbidity, and outcomes.[
Furthermore, patient selection criteria are an important step in the procedure of minimally invasive endoscopic evacuation of ICH to achieve a favorable outcome. We conducted a retrospective analysis of endoscopically treated patients in our department. The encouraging results presented below support the decision to convert to endoscopy for selected patients at our institution.
MATERIALS AND METHODS
Study design
This retrospective STROBE-compliance[
Study population
This study was conducted at a primary tertiary hospital. From March 2018 to March 2020, 20 patients were considered for endoscopic evacuation of intracerebral hemorrhage. Six patients with intraventricular hematoma (IVH), ten with intraparenchymal (IP) hematoma, and four with IP hematoma with intraventricular extension (IP&IVH) were included in this study. All patients benefited from an on-site head computed tomography (CT) scan with systematic CT angiography (CTA) for etiological findings and further exploration with digital subtraction angiography (DSA) in cases with non-conclusive CTA. In this study, we classified the hematoma evacuation rate as low (<70% evacuated), moderate (70–90% evacuated), and high (>90% evacuated). The surgical outcome was also divided into three groups (favorable, mRS 0–2; unfavorable, mRS 3–5; death, mRS 6) for clarity.
Patient selection for endoscopic minimally invasive intracranial hematoma evacuation (MIHE)
Inclusion criteria
The study only included
Patients: ≤75 years old, with a Glasgow coma scale (GCS) score >8, presenting within 48 h of onset. Lesion: Lobar, putaminal, thalamic, intraventricular, and deep-seated hematomas ≥ 30mL in volume (calculated on the CT scan at admission based on the Tada formula). Anticoagulant therapy: All patients undergoing anticoagulant treatment were considered for surgery after normalization of coagulation parameters. This medical treatment is done according to the protocol and depends on the patient’s underlying condition and the type of anticoagulant drug.
Patient selection criteria represent important factors that can easily influence surgical outcomes, regardless of surgical procedure and surgeon skill. Therefore, the inclusion criteria for minimally invasive endoscopic ICH evacuation are highlighted in this study.
The first and most obvious inclusion criterion that most authors agreed on was CT-confirmed ICH diagnosis. Based on the approximate ellipse volume, the volume of the ICH in milliliters was calculated using the formula A × B × C/2, where A is the largest hematoma diameter on axial CT slices in centimeters, and B is the hematoma diameter perpendicular to the same slice. C is the number of CT slices with visible hematomas multiplied by the slice thickness (cm).[
The GCS score at admission was the second inclusion criterion for all authors, with a commonly reported GCS score of >8.[
The third inclusion criterion was patient age. Most authors have specified an age limit of 65 years,[
The fifth inclusion criterion, for which some inconsistencies have been noted in the literature, was the duration of bleeding from stroke onset to surgery. Some studies included patients with a hemorrhage duration of 24 h,[
Exclusion criteria
Were excluded from this study all the patients with
Etiologic findings on the CTA or DSA, Hemorrhagic conversion of the cerebral infarction, Downregulation of the GCS following admission with a rebleeding on a CT scan.
Surgical technique
The patients were operated on in a supine position under general anesthesia. The appropriate entry point was determined based on the location of the hematoma in relation to the eloquent areas, following its long axis whenever possible. As no navigation system was available in the emergency operating room, superficial lobar hematomas were approached based on the calculation of anatomical landmarks [
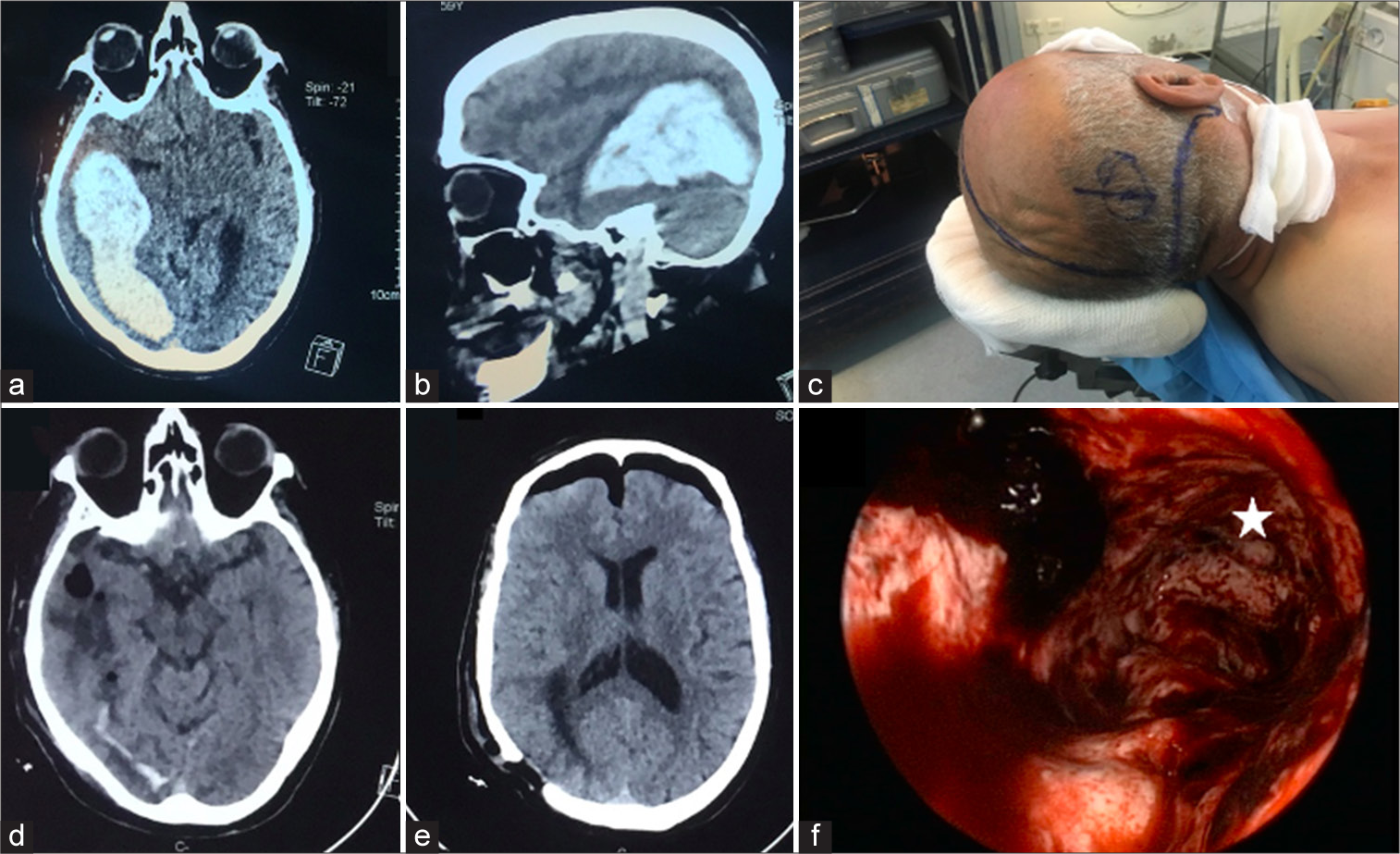
Figure 1:
Case illustration of a large intraparenchymal lobar hematoma: Preoperative computed tomography scan on (a) axial and (b) sagittal images showing the occipitotemporal extension of the hematoma. (c) The patient was positioned in a supine position, with marked anatomical landmarks and entry point calculation. (d and e) Immediate postoperative CT scan showing the complete evacuation of the hematoma; note the entry point on (e). (f) Operative view of the hematoma cavity, the deepest part (*) representing the anterior temporal limit of the cavity.
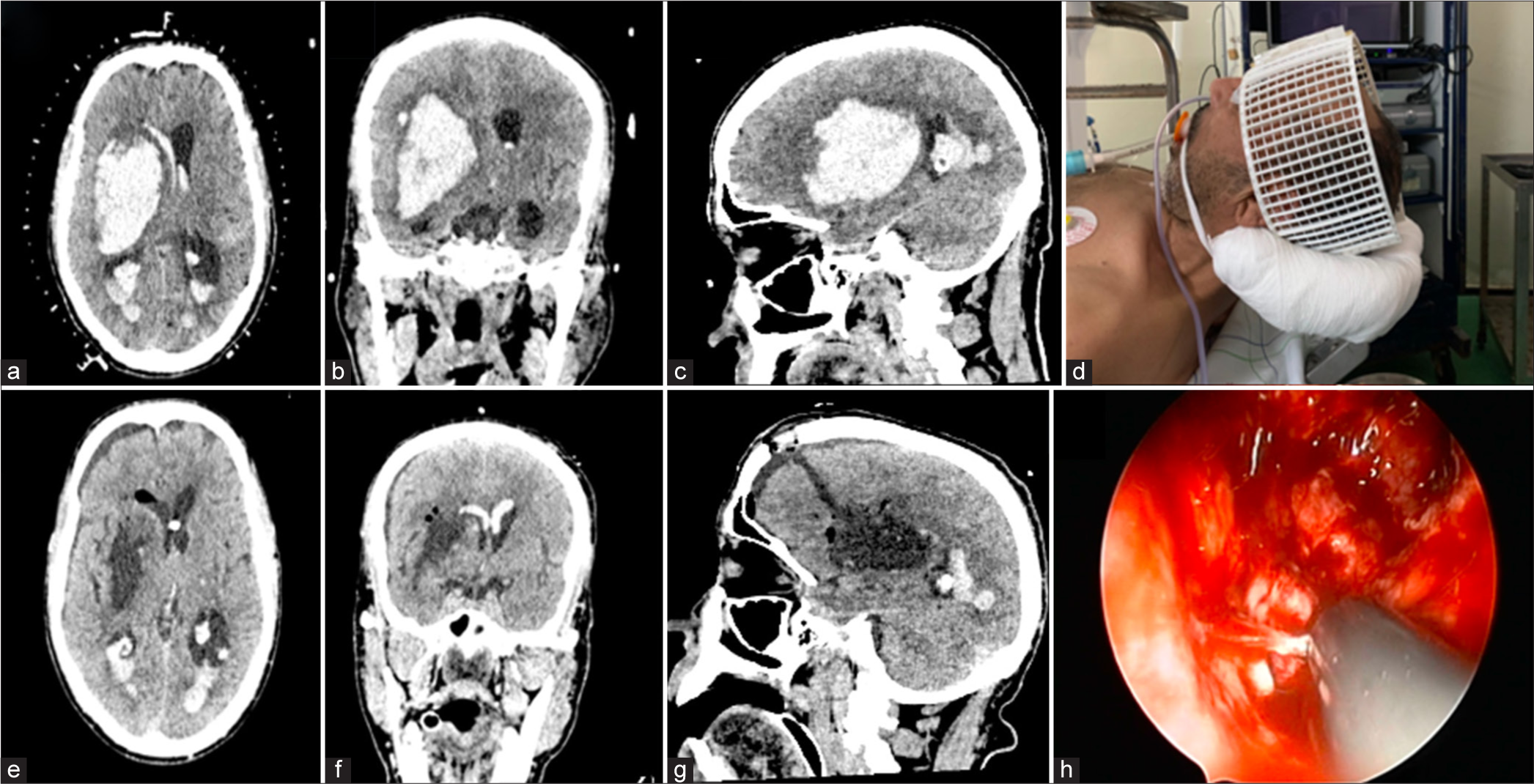
Figure 2:
Case illustration of a large capsular hematoma breaking into the ventricles: (a) Preoperative computed tomography scan on axial (b) coronal and (c) sagittal images. Note the Craniomapper™ reference letters (a) that help in entry point calculation. (d) Photomicrograph of the craniomapper system. (e-g) Postoperative CT scan showing complete evacuation of the hematoma and external ventricular drainage placement (e). Note the entry point and trajectory on (g). (h) Operative view of the cavity and coagulation of a bleeding perforator with the combined suction-coagulation cannula.
A linear (or curvilinear) incision was made, and a 2 cm diameter burr-hole was drilled [
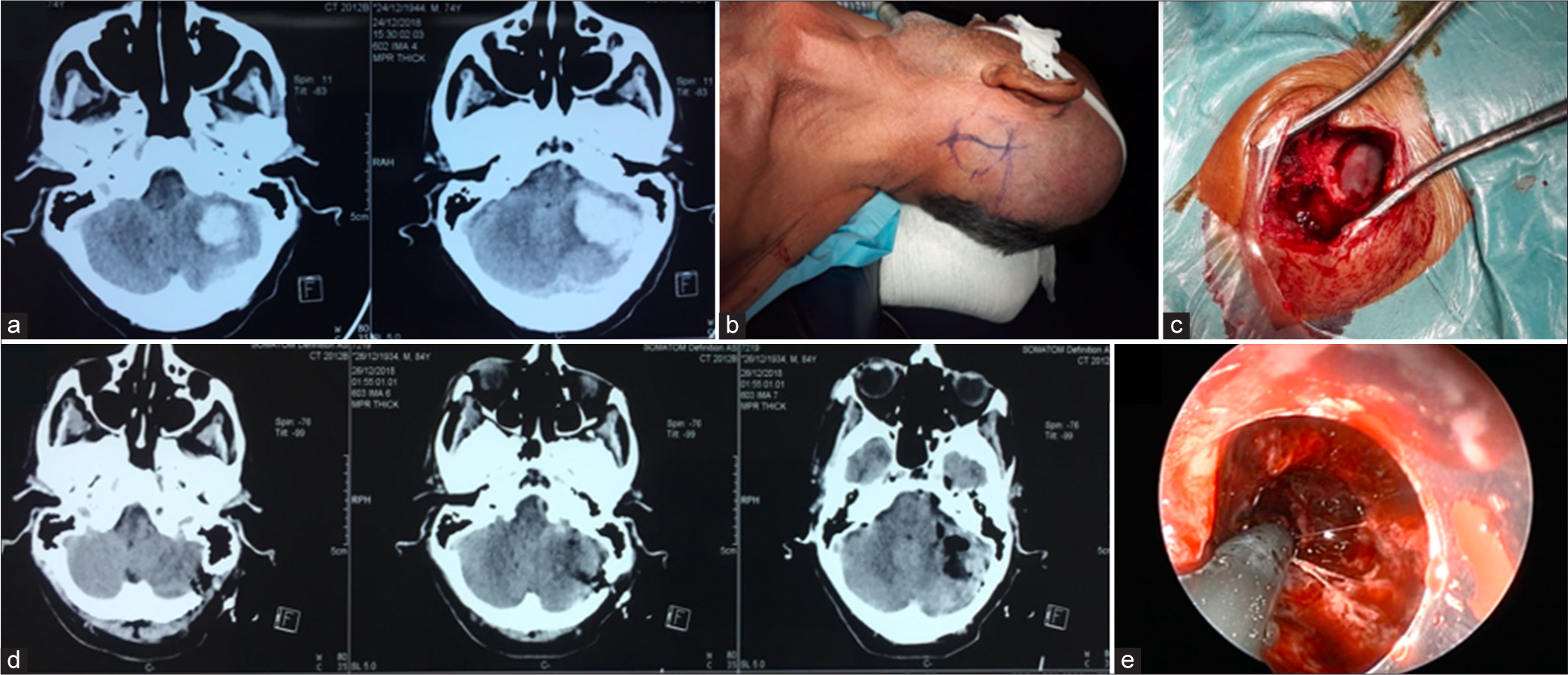
Figure 3:
(a) Case illustration of a cerebellar hematoma preoperative computed tomography (CT) scan on axial images and (b) patient supine positioning with entry point and incision markings . (c) Photomicrograph of the bone window. (d) Immediate postoperative CT scan showing the entry point and the cleaned hematoma cavity. (e) Operative view from inside the transparent sheath with coagulation of bleeding vessels using the combined suction-coagulation cannula.
Three types of transparent sheets were alternatively used: the 6 mm in diameter Nishihara-type sheath,[
All operations were performed by the same surgical team (MYO, HB, and AEO), who had extensive experience in various endoscopic procedures (ventricular and skull base endoscopy) and had attended several workshops and visited the centers where the procedure was performed. This allowed us to monitor the evolution of the learning curve and ensure better coordination between operators. Once the hematoma was reached, one surgeon held the endoscope and controlled the direction and course of the sheath, whereas the other surgeon controlled the suction cannula, coagulation, and irrigation.
The hematoma was approached along its long axis, and suction began at the center of the hematoma and progressed as the hematoma was pushed into the sheath by the elevated ICP,[
Cases of intraventricular hemorrhage were approached through the standard Kaucher point with a 2 × 3 cm egg-shaped burr-hole. In the case of bilateral hemorrhage, the ventricle with the largest hematoma was chosen as access, and a septostomy was created after ipsilateral aspiration to reach the contralateral hematoma. Again, the wet field technique helped dilate the ventricles, wash out the ventricular walls, and mobilize distal blood clots near the suction. At the end of the procedure, a ventricular catheter was inserted through the foramen of Monro under visual control.
In the postoperative period, serial CT scans were performed (immediately and on days 2 and 5) to monitor the evacuation rate and to check for possible rebleeding. Records of all surgeries and patient demographics were reviewed to document the duration of surgery, preoperative and postoperative hematoma volume, shunt duration (for ventricular hemorrhage), clinical outcome (Glasgow Outcome Scale [GOS] and mRS), and complications.
The pre-and post-operative hematoma volumes were calculated based on the simple ABC/2 formula for IP hematomas and the IVH score for intraventricular hemorrhage.[
Data analysis
All statistical analyses were performed using JAMOVI version 2.3.0, with the significance level set at P ≤ 0.05. First, we conducted a descriptive analysis of the data. An independent Student’s t-test was used to evaluate the continuous and ordinal variables. Chi-square or Fisher’s exact test was used to compare categorical variables. Oneway analysis of variance was used to assess any significant differences between independent variables in terms of age or outcome (dependent variable). Multivariate analysis was used to test their effects on the outcome. The homogeneity of variance was set for Levene’s test at P < 5%, with a low P-value suggesting a violation of the normality assumption.
RESULTS
Study characteristics
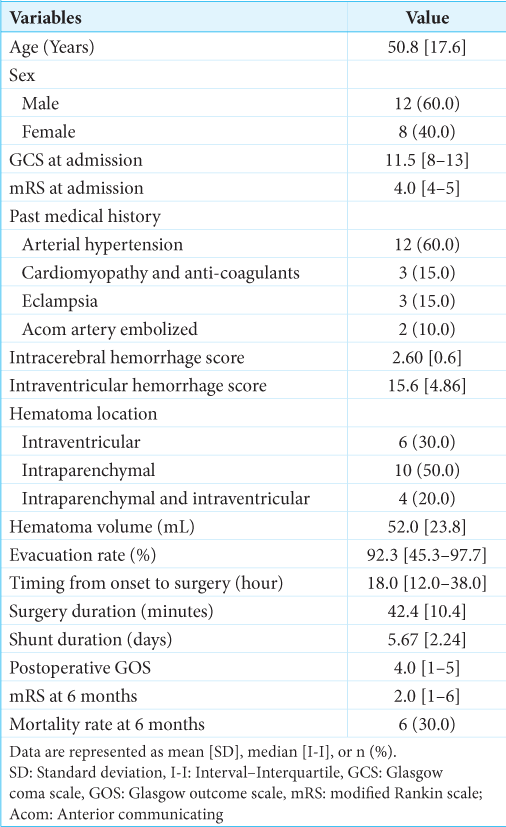
From March 2018 to May 2020, 20 patients underwent endoscopic evacuation for IP hematoma, IVH, and IP&IVH. The mean age was 50.8 [17.6] years, with a male predominance of 60.0% (n = 12). The past medical history included arterial hypertension (60.0%, n = 12), cardiomyopathy with anticoagulants (15.0%, n = 3), eclampsia (15.0%, n = 3), and anterior communicating artery embolization (10.0%, n = 2). The median GCS and mRS scores at admission were 11.5 [8–13] and 4.0 [4–5], respectively. The overall mean intracerebral hemorrhage score and intraventricular hemorrhage score were 2.60 [0.6] and 15.6 [4.86], respectively [
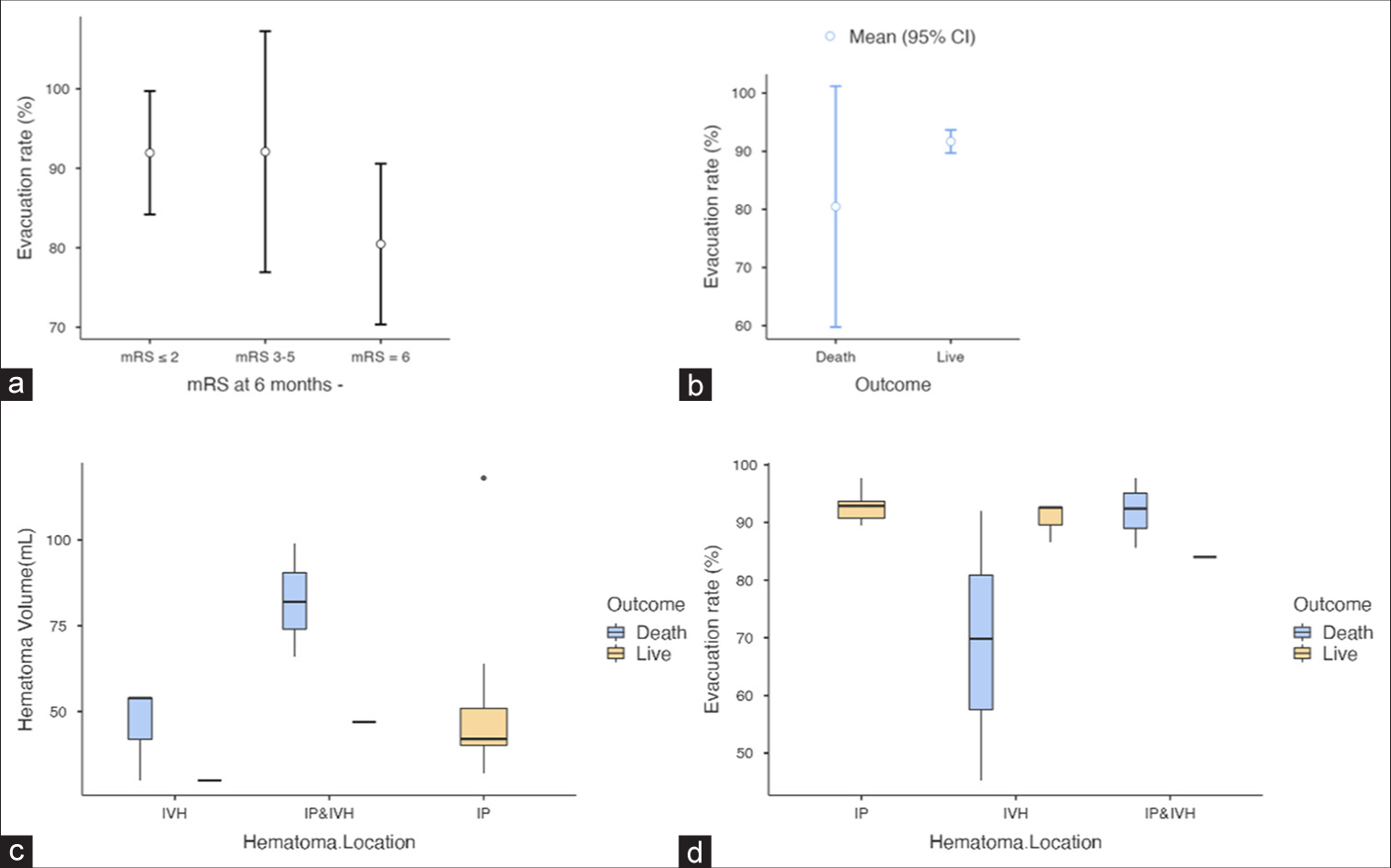
Figure 4:
(a and b) are descriptive plots for the relation between the modified Rankin scale at 6 months and the outcome by the hematoma evacuation rate. (c and d) Box plots for the association between the outcome and hematoma volume hematoma evacuation rate by its location. CI: Confidence interval, mRS: Modified Rankin scale, IVH: Intraventricular hemorrhage.
IP hematoma patients
Ten patients (50.0%) were considered for endoscopic evacuation of an IP hemorrhage. The IP hematoma was lobar in 6 cases (30.0%), putaminal and deep-seated in 2 cases (10.0%), and cerebellar in 2 cases (10.0%). Compared to the other two hematoma locations, the IP patient was admitted with the highest GCS (12.0, 95% confidence interval [CI] [11.33–12.67]). The mean surgical duration was 39.90 min (95% CI [32.52–47.28]), the mean mRS of (4.0, 95% CI [4.0–5.0]), and the mean hematoma volume was 51.9 mL (95% CI [34.17–69.62]), with a high evacuation rate (92.78%, 95% CI [90.99–94.57]). The mRS at 6 months follow-up was (1.90, 95% CI [1.37–2.43]) for this group [
IVH patients
Six (30.0%) patients were considered for endoscopic evacuation of a primary massive ventricular hemorrhage. The IVH patient was admitted with a mean GCS score (9.83, 95% CI [7.59–12.08]), a mean mRS of (4.50, 95% CI [3.92–5.07]), and a mean hematoma volume of 38.0 mL (95% CI [25.0–51.01]), with a moderate evacuation rate compared to the previous group (79.82%, 95% CI [59.83–99.80]). The mean surgical duration was 44.67 min (95% CI [38.04–51.29]), and the mRS at 6 months follow-up was (4.17, 95% CI [2.02–6.31]). Half of these patients (50%, n = 3) died after surgery for causes that might not be related to minimally invasive endoscopic hematoma evacuation [
IP&IVH patients
In contrast, IVH secondary to IP hematoma was more deadly than the two previous hematoma locations. The intracranial volume of blood was more important (73.50 mL, 95% CI [38.14–105.85]) with a moderate evacuation rate (89.92%, 95% CI [79.84–100.01]). The mortality rate was 75% [
DISCUSSION
Main findings
Following endoscopic MIHE, a high evacuation rate (>90%) was significantly associated with a favorable outcome (mRS ≤2) regardless of the location and volume of the hematoma. The overall mortality rate was 30% but varied greatly depending on the location of the hematoma: IP hematoma (0%), IVH (50%), and IP and IVH (75%). The presence of blood in the ventricles is significantly associated with a high mortality rate.
Neuroendoscopic practice and implementation for MIHE
Since its first description by Auer et al. in 1989, endoscopic hematoma evacuation has been aimed at overcoming classical surgical trauma while ensuring safe maximal evacuation under visual control.[
Further developments in endoscopic technology over the past few decades have helped to standardize this technique and improve its safety and efficacy.
One of the major improvements was the introduction of the transparent sheath by Nishihara et al.,[
In the present study, we used three different types of transparent sheaths depending on their availability under our conditions and gave our impression of their usefulness:
The Nishihara sheath is a reusable rigid working channel with a metallic inner style that is withdrawn once the hematoma is reached. It has round atraumatic edges and offers clear visualization. Its 10 cm length makes it suitable for intraventricular hemorrhage evacuation. However, its 6 mm inner diameter provides little room for instrument manipulation, making it more useful for experienced surgeons. The Neuroport system (Neuroport® Olympus, Japan) is a single-use soft sheath with a cannulated inner stylet that allows endoscopic introduction and direct visualization of advancement through the brain parenchyma until the hematoma is reached. The inner stylet was then withdrawn. The 10 mm inner diameter is large enough for manipulation, and the soft wall of the sheath is parenchyma-friendly. The length was also adequate for intraventricular hemorrhage evacuation. The regular syringe-based sheath is the most cost-effective system, as described by many authors,[
Most authors used an 18 cm length endoscope with various diameters (ranging from 2.7 to 4 mm) and angles (0° being the most popular).[
Our surgical settings were different from those described previously. While most authors recommend that the main surgeon holds both the endoscope and suction cannula,[
IP hematoma evacuation
Our evacuation technique mainly followed Kuo et al.,[
As part of the basic technique and due to the characteristics of the endoscope and working channel, the long axis of the hematoma determines the selection of the entry point for lobar hematomas. The same principle can be applied to deep-seated hematomas because most have an elliptical shape, leading to the choice of the frontal approach.[
In addition to achieving high evacuation rates due to advantageous visual control, another highlighted advantage of endoscopic hematoma evacuation is the reduced operation time. In the present study, the mean duration of endoscopic evacuation of IP was 39.90 min (95% CI [32.52–47.28]). This operative time was influenced by the learning curve, where appropriate training is required to transition to an endoscopic technique.[
In 2011, Kuo et al.[
IVH evacuation
The rationale for endoscopic IVH evacuation in a highly fatal pathology is to restore CSF circulation pathways and control intracranial pressure.[
In our experience, evacuation of IVHs was initiated after sufficient control of the endoscopic technique and was surgically more demanding. Although the ventricular approach is more conventional and blood clots are much easier to aspirate, the vulnerability of the ventricular walls and structures requires more skill and attention. Further difficulties were related to the progressive collapse of the ventricular walls during evacuation under dry field aspiration,[
Nevertheless, in most cases, high evacuation rates were achieved with minimal invasiveness, and the learning curve improved rapidly. The procedure duration depended mainly on the need for additional contralateral access. The mean operation time was 44.67 min (95% CI [38.04–51.29]). A shorter shunt duration is also an expected outcome of endoscopic ventricular washout, reducing shunt-related infections.[
Study limitations
This study has some limitations that should be acknowledged. The first reason is its retrospective nature and the limited number of patients. In addition, patients who underwent surgery within 48 h of ictus and those who benefited from normalization of coagulation parameters were included in the study. These patients usually have poor prognoses. This may explain why our mortality rate was slightly higher than that reported in the literature. Therefore, good surgical outcomes depend on patient selection. However, further randomized controlled trials are required to demonstrate the safety and effectiveness of endoscopic techniques.
CONCLUSION
High evacuation rates of IP and IVH s can be achieved within a short operation time, with minimal additional morbidity and mortality during endoscopy. With good patient selection, sufficient knowledge and training in the handling and use of endoscopes, and good coordination between operators, the learning curve can be achieved in the first few cases. This study also provides detailed descriptions and useful information for the implementation of this technique.
Ethical approval:
The Institutional Review Board approval is not required as it is retrospective study . This retrospective study was conducted according to the tenets of the Helsinki Declaration.
Declaration of patient consent:
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship:
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Ali M, Yaeger K, Ascanio L, Troiani Z, Mocco J, Kellner CP. Early minimally invasive endoscopic intracerebral hemorrhage evacuation. World Neurosurg. 2021. 148: 115
2. Auer LM, Deinsberger W, Niederkorn K, Gell G, Kleinert R, Schneider G. Endoscopic surgery versus medical treatment for spontaneous intracerebral hematoma: A randomized study. J Neurosurg. 1989. 70: 530-5
3. Bakshi A, Bakshi A, Banerji AK. Neuroendoscope-assisted evacuation of large intracerebral hematomas: Introduction of a new, minimally invasive technique. Preliminary report. Neurosurg Focus. 2004. 16: e9
4. Basaldella L, Marton E, Fiorindi A, Scarpa B, Badreddine H, Longatti P. External ventricular drainage alone versus endoscopic surgery for severe intraventricular hemorrhage: A comparative retrospective analysis on outcome and shunt dependency. Neurosurg Focus. 2012. 32: E4
5. Beck J, Fung C, Strbian D, Bütikofer L, Z’Graggen WJ, Lang MF. Decompressive craniectomy plus best medical treatment versus best medical treatment alone for spontaneous severe deep supratentorial intracerebral haemorrhage: A randomised controlled clinical trial. Lancet. 2024. 403: 2395-404
6. Chen CC, Liu CL, Tung YN, Lee HC, Chuang HC, Lin SZ. Endoscopic surgery for intraventricular hemorrhage (IVH) caused by thalamic hemorrhage: Comparisons of endoscopic surgery and external ventricular drainage (EVD) surgery. World Neurosurg. 2011. 75: 264-8
7. Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019. 13: S31-4
8. Du B, Shan AJ, Peng YP, Wang J, Peng KW, Zhong XL. A new modified neuroendoscope technology to remove severe intraventricular haematoma. Brain Inj. 2018. 32: 1142-8
9. Falcone J, Chen JW. Early minimally invasive parafascicular surgery for evacuation of spontaneous intracerebral hemorrhage in the setting of computed tomography angiography spot sign: A case series. Oper Neurosurg (Hagerstown). 2022. 22: 123-30
10. Fiorella D, Arthur A, Bain M, Mocco J. Minimally invasive surgery for intracerebral and intraventricular hemorrhage: Rationale, review of existing data and emerging technologies. Stroke. 2016. 47: 1399-406
11. Fiorindi A, Saraceno G, Zanin L, Terzi di Bergamo L, Feletti A, Doglietto F. Endoscopic evacuation of massive intraventricular hemorrhages reduces shunt dependency: A meta-analysis. Asian J Neurosurg. 2022. 17: 541-6
12. Greenberg SM, Ziai WC, Cordonnier C, Dowlatshahi D, Francis B, Goldstein JN. 2022 Guideline for the management of patients with spontaneous intracerebral hemorrhage: A guideline from the American Heart Association/American Stroke Association. Stroke. 2022. 53: e282-361
13. Gregson BA, Broderick JP, Auer LM, Batjer H, Chen XC, Juvela S. Individual patient data subgroup meta-analysis of surgery for spontaneous supratentorial intracerebral hemorrhage. Stroke. 2012. 43: 1496-504
14. Gregson BA, Rowan EN, Mendelow AD, editors. Letter to the editor by Gregson et al regarding article, “minimally invasive surgery for spontaneous supratentorial intracerebral hemorrhage: A meta-analysis of randomized controlled trials”. Stroke. 2013. 44: e45
15. Hallevi H, Dar NS, Barreto AD, Morales MM, Martin-Schild S, Abraham AT. The IVH score: A novel tool for estimating intraventricular hemorrhage volume: Clinical and research implications. Crit Care Med. 2009. 37: 969-74.e1
16. Hamada H, Hayashi N, Kurimoto M, Umemura K, Nagai S, Kurosaki K. Neuroendoscopic removal of intraventricular hemorrhage combined with hydrocephalus. Minim Invasive Neurosurg. 2008. 51: 345-9
17. Hanley DF, Thompson RE, Rosenblum M, Yenokyan G, Lane K, McBee N. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): A randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. 2019. 393: 1021-32
18. Hannah TC, Kellner R, Kellner CP. Minimally invasive intracerebral hemorrhage evacuation techniques: A review. Diagnostics (Basel). 2021. 11: 576
19. Hayashi T, Karibe H, Akamatsu Y, Narisawa A, Shoji T, Sasaki T. Endoscopic hematoma evacuation for intracerebral hemorrhage under local anesthesia: Factors that affect the hematoma removal rate. World Neurosurg. 2019. 126: e1330-6
20. Hemphill JC, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M. Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015. 46: 2032-60
21. Komatsu F, Komatsu M, Wakuta N, Oshiro S, Tsugu H, Iwaasa M. Comparison of clinical outcomes of intraventricular hematoma between neuroendoscopic removal and extraventricular drainage. Neurol Med Chir (Tokyo). 2010. 50: 972-6
22. Kuo LT, Chen CM, Li CH, Tsai JC, Chiu HC, Liu LC. Early endoscope-assisted hematoma evacuation in patients with supratentorial intracerebral hemorrhage: Case selection, surgical technique, and long-term results. Neurosurg Focus. 2011. 30: E9
23. Li Y, Zhang H, Wang X, She L, Yan Z, Zhang N. Neuroendoscopic surgery versus external ventricular drainage alone or with intraventricular fibrinolysis for intraventricular hemorrhage secondary to spontaneous supratentorial hemorrhage: A systematic review and meta-analysis. PLoS One. 2013. 8: e80599
24. Li Y, Yang R, Li Z, Yang Y, Tian B, Zhang X. Surgical evacuation of spontaneous supratentorial lobar intracerebral hemorrhage: Comparison of safety and efficacy of stereotactic aspiration, endoscopic surgery, and craniotomy. World Neurosurg. 2017. 105: 332-40
25. Longatti P, Basaldella L. Endoscopic management of intracerebral hemorrhage. World Neurosurg. 2013. 79: S17-e1-7
26. Ma L, Hou Y, Zhu R, Chen X. Endoscopic evacuation of basal ganglia hematoma: Surgical technique, outcome, and learning curve. World Neurosurg. 2017. 101: 57-68
27. Morgenstern LB, Hemphill JC, Anderson C, Becker K, Broderick JP, Connolly ES. Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010. 41: 2108-29
28. Nagasaka T, Tsugeno M, Ikeda H, Okamoto T, Inao S, Wakabayashi T. Early recovery and better evacuation rate in neuroendoscopic surgery for spontaneous intracerebral hemorrhage using a multifunctional cannula: Preliminary study in comparison with craniotomy. J Stroke Cerebrovasc Dis. 2011. 20: 208-13
29. Nishihara T, Morita A, Teraoka A, Kirino T. Endoscopy-guided removal of spontaneous intracerebral hemorrhage: Comparison with computer tomography-guided stereotactic evacuation. Childs Nerv Syst. 2007. 23: 677-83
30. Nishihara T, Teraoka A, Morita A, Ueki K, Takai K, Kirino T. A transparent sheath for endoscopic surgery and its application in surgical evacuation of spontaneous intracerebral hematomas. Technical note. J Neurosurg. 2000. 92: 1053-5
31. Oertel JM, Mondorf Y, Baldauf J, Schroeder HW, Gaab MR. Endoscopic third ventriculostomy for obstructive hydrocephalus due to intracranial hemorrhage with intraventricular extension. J Neurosurg. 2009. 111: 1119-26
32. Orakcioglu B, Beynon C, Bösel J, Stock C, Unterberg AW. Minimally invasive endoscopic surgery for treatment of spontaneous intracerebral hematomas: A single-center analysis. Neurocrit Care. 2014. 21: 407-16
33. Pan J, Chartrain AG, Scaggiante J, Spiotta AM, Tang Z, Wang W. A Compendium of modern minimally invasive intracerebral hemorrhage evacuation techniques. Oper Neurosurg (Hagerstown). 2020. 18: 710-20
34. Pantazis G, Tsitsopoulos P, Mihas C, Katsiva V, Stavrianos V, Zymaris S. Early surgical treatment vs conservative management for spontaneous supratentorial intracerebral hematomas: A prospective randomized study. Surg Neurol. 2006. 66: 492-501
35. Pradilla G, Ratcliff JJ, Hall AJ, Saville BR, Allen JW, Paulon G. Trial of Early minimally invasive removal of intracerebral hemorrhage. N Engl J Med. 2024. 390: 1277-89
36. Rothrock RJ, Chartrain AG, Scaggiante J, Pan J, Song R, Hom D. Advanced techniques for endoscopic intracerebral hemorrhage evacuation: A technical report with case examples. Oper Neurosurg (Hagerstown). 2020. 20: 119-29
37. Song P, Duan FL, Cai Q, Wu JL, Chen XB, Wang Y. Endoscopic surgery versus external ventricular drainage surgery for severe intraventricular hemorrhage. Curr Med Sci. 2018. 38: 880-7
38. Sun GC, Chen XL, Hou YZ, Yu XG, Ma XD, Liu G. Image-guided endoscopic surgery for spontaneous supratentorial intracerebral hematoma. J Neurosurg. 2017. 127: 537-42
39. Tyebkhan G. Declaration of Helsinki: The ethical cornerstone of human clinical research. Indian J Dermatol Venereol Leprol. 2003. 69: 245-7
40. Wang W-H, Hung Y-C, Hsu SP, Lin C-F, Chen H-H, Shih Y-H. Endoscopic hematoma evacuation in patients with spontaneous supratentorial intracerebral hemorrhage. J Chin Med Assoc. 2015. 78: 101-7
41. Xu X, Chen X, Li F, Zheng X, Wang Q, Sun G. Effectiveness of endoscopic surgery for supratentorial hypertensive intracerebral hemorrhage: A comparison with craniotomy. J Neurosurg. 2018. 128: 553-9
42. Yamamoto T, Nakao Y, Mori K, Maeda M. Endoscopic hematoma evacuation for hypertensive cerebellar hemorrhage. Minim Invasive Neurosurg. 2006. 49: 173-8
43. Yao Z, Hu X, You C, He M. Effect and feasibility of endoscopic surgery in spontaneous intracerebral hemorrhage: A systematic review and meta-analysis. World Neurosurg. 2018. 113: 348-56.e2
44. Zhao YN, Chen XL. Endoscopic treatment of hypertensive intracerebral hemorrhage: A technical review. Chronic Dis Transl Med. 2016. 2: 140-6
45. Zhou X, Chen J, Li Q, Ren G, Yao G, Liu M. Minimally invasive surgery for spontaneous supratentorial intracerebral hemorrhage: A meta-analysis of randomized controlled trials. Stroke. 2012. 43: 2923-30
46. Zhu H, Wang Z, Shi W. Keyhole endoscopic hematoma evacuation in patients. Turk Neurosurg. 2012. 22: 294-9
47. Zhu J, Tang C, Cong Z, Yang J, Cai X, Liu Y. Endoscopic intraventricular hematoma evacuation surgery versus external ventricular drainage for the treatment of patients with moderate to severe intraventricular hemorrhage: A multicenter, randomized, controlled trial. Trials. 2020. 21: 640