- Department of Neurosurgery, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia
- Department of Anatomical Pathology, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia
Correspondence Address:
Ahmad Faried, Department of Neurosurgery, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia.
DOI:10.25259/SNI_881_2024
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ahmad Faried1, Edward Jaya Hadi2, Hasrayati Agustina2. Poor diagnostic value of isocitrate dehydrogenase 1 R132H immunohistochemistry for determination of isocitrate dehydrogenase 1 status in patients with glioblastoma. 18-Apr-2025;16:140
How to cite this URL: Ahmad Faried1, Edward Jaya Hadi2, Hasrayati Agustina2. Poor diagnostic value of isocitrate dehydrogenase 1 R132H immunohistochemistry for determination of isocitrate dehydrogenase 1 status in patients with glioblastoma. 18-Apr-2025;16:140. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13509
Abstract
BackgroundThe World Health Organization (WHO) classification of central nervous system (CNS) tumors is a major advance toward improving the diagnosis of adult brain tumors. Despite the promise of isocitrate dehydrogenase (IDH) mutations as an important biomarker for glioblastoma, not all institutions have ready access to mutation detection polymerase chain reaction (PCR) methods, and deoxyribonucleic acid (DNA) sequencing may be problematic in very small biopsies. However, a simultaneous evaluation of IDH1 status by DNA sequencing and immunohistochemistry (IHC) to determine the sensitivity and specificity of both methods, along with their predictive value, was unavailable.
MethodsThis retrospective study included 33 patients who underwent surgical resection or biopsy, January 2016–December 2019. The diagnosis of glioblastoma was established. Surgically resected tumor tissues were fixated in 10%-formaldehyde preserved in paraffin-embedded blocks. Glioblastoma was classified according to the 2021 WHO classification of CNS tumors. The enrolled patients were followed up to obtain the overall survival rate (median follow-up time, 30 months).
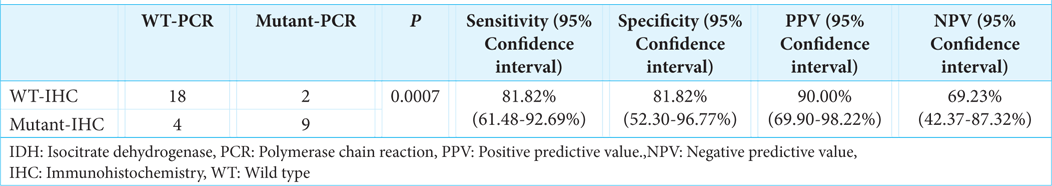
ResultsThirty-three patients (14 male; 19 female), mean age of 44.74 ± 15.49 years. Eight had WHO Grade II, 2 with WHO Grade III, and 23 with WHO Grade IV. The sensitivity and specificity of IDH1 IHC were 81.82% (P = 0.0007), a positive predictive value of 90.00% (69.90-98.22%), and a negative predictive value of 69.23% (42.37-87.32%). The survival rate was significantly higher in IDH1 mutant than wild-type IDH1, whether based on IHC or PCR (P = 0.0014).
ConclusionIDH1 status evaluation is crucial to predicting the survival rate and important for guiding the treatment decision for patients with glioblastoma. Despite the lesser sensitivity and specificity of IHC in comparison to DNA sequencing in this study, larger prospective studies are needed to validate our preliminary finding.
Keywords: Central nervous system tumor, Glioblastoma, Immunohistochemistry, Isocitrate dehydrogenase 1 IDH1 R132H, Polymerase chain reaction
INTRODUCTION
The new version of the World Health Organization (WHO) classification of central nervous system (CNS) tumors is a significant progress in improving the identification of adult brain tumors.[
Wild-type IDH1 is an important metabolic enzymes that catalyze the oxidative decarboxylation of isocitrate to generate α-ketoglutarate (αKG) and carbon dioxide that play a role in malignancy. The common function of IDH1 active-site mutation is a neomorphic enzyme activity catalyzes the conversion of αKG to D-2-hydroxyglutarate (D2HG). Under physiological conditions, cellular D2HG accumulation is limited due to the actions of the endogenous D2HG dehydrogenase, which catalyzes the conversion of D2HG to αKG. However, the neomorphic activity of mutant IDH causes D2HG to accumulate to supraphysiological levels within cells. Elevated D2HG concentrations can be detected in the serum of patients with IDH-mutant gliomas in patients.[
IDH1 immunohistochemistry (IHC) can detect cancer cells with mutations by utilizing an antibody specific to the prevalent R132H mutant variant of IDH1.[
MATERIALS AND METHODS
Subjects
The Committee of Ethics of the Faculty of Medicine of Padjadjaran University provided ethical approval. This retrospective study comprised 33 patients who had surgical resection or biopsy from January 2016 to December 2019, and the diagnosis of primary glioblastoma was confirmed. Tissues from surgically excised tumors were fixed in 10% formaldehyde and implanted in paraffin blocks. Glioblastoma was classified and graded based on the 2021 WHO classification of CNS cancers.[
IDH1 IHC
Slides of tumor tissue with a thickness of four microns were deparaffinized and rehydrated. Antigen retrieval was carried out using a decloaking chamber (DC2008INTL; Biocare Medical, Pacheco, CA, USA) at 100°C for 20 min using an antigen retrieval solution (Tris ethylenediaminetetraacetic acid 10 mmol/L, pH 9.0). After cooling at room temperature, sections were washed 2× with phosphate-buffered saline (PBS) for 5 min each. Endogenous peroxidase activity was stopped by dipping sections in 3% hydrogen peroxide blocker (Boster Biological Technology, Pleasanton, CA, USA) for 10 min and rinsed in three changes of PBS. Following the initial processing step, sections were incubated at room temperature with primary antibodies anti-human IDH1 R132H mutant specific AB (GTX57185 Genetex, IHC 132, mouse monoclonal AB, at 1:200 dilution), followed by 30 min of incubation with the poly horseradish peroxidase (HRP) non-biotin detection system. Finally, the sections were counterstained with hematoxylin and eosin, then dehydrated and mounted. Positive results revealed strong cytoplasmic exclusively in tumor cells, while negative controls were produced concurrently for all 33 samples by replacing the primary AB with distilled water.
Statistical analysis
We used GraphPad Prism v8.0 for the statistical analysis. P < 0.05 was considered as statistically significant.
RESULTS
Subjects characteristic
This study included 33 patients (14 male and 19 female), with a mean age of 44.74 ± 15.49 years old. According to the 2021 WHO categorization with PCR examination, the participants included eight patients with Grade II, two patients with Grade III, and 23 patients with Grade IV.
The reliability of IHC
Direct DNA sequencing validated 23 IDH-wild type and 10 IDH1 gene mutant samples [
Survival rate
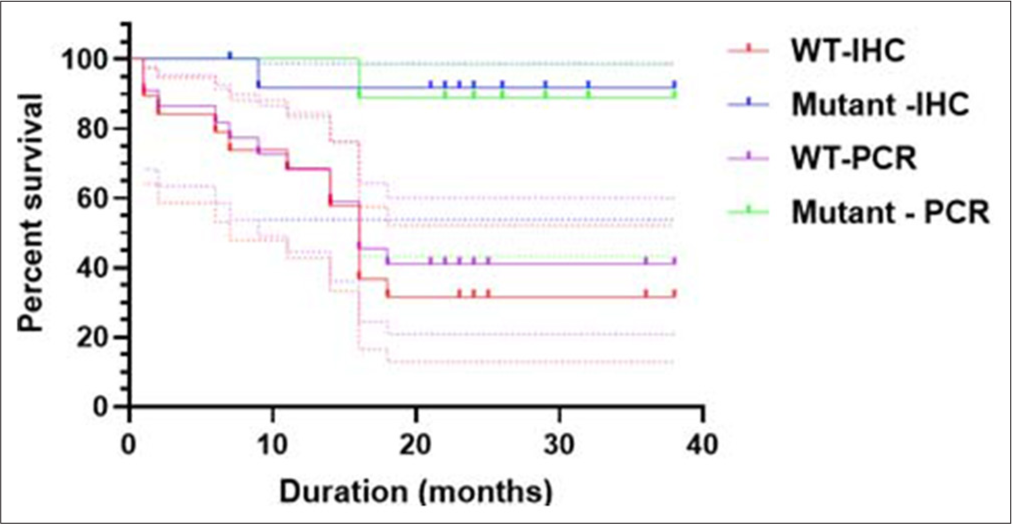
In this study, we discovered that patients with IDH1 mutants had a greater survival rate than patients with wild-type IDH1, whether using IHC or PCR [
Figure 3:
The survival rate of patients with IDH1 status. Patients with IDH1 mutant have a higher survival rate than patients with gliomas with wild-type IDH1 status, whether based on the immunohistochemistry or PCR (Log rank mantle cox, P = 0.0014). IDH1: Isocitrate dehydrogenase 1, PCR: Polymerase chain reaction. WT: Wild type, WT-IHC :Wild type-immunohistochemistry.
DISCUSSION
Malignant gliomas are fundamentally a genetic disease that shares characteristics with nearly all human cancers.[
The assessment of IDH1 status is critical for diagnosis and developing an effective treatment strategy. This can be performed either through DNA sequencing or IHC.[
In a study comparing IHC to genetic testing, Sporikova et al., discovered that IHC was 100% sensitive and specific for detecting IDH1-R132H mutations, demonstrating that anti-IDH1-R132H immunostaining is a trustworthy technique for assessing the status of IDH1 gene mutations.[
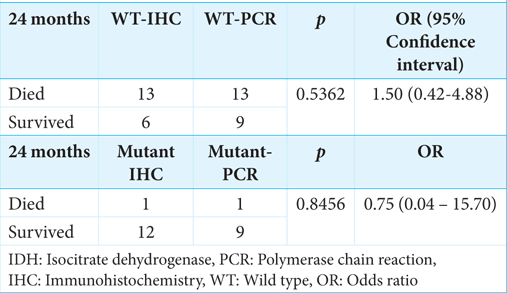
Either based on IHC or PCR, we discovered that the survival rate was significantly higher (P = 0.0014) in patients with IDH1 mutant than in patients with IDH1 wild-type [
In our cohort, we found no difference in survival rates between the IHC and sequencing-detected IDH1 mutants; this impact could be related to the small number of samples. Despite its reduced sensitivity and specificity compared to DNA sequencing, IHC is beneficial for detecting the IDH1 R132H mutation, is less expensive, and is more easily available in countries with limited resources. However, the correct identification of mutation status in diffuse gliomas is crucial for identifying appropriate tailored therapies and adhering to the latest 2021 WHO system, which is based on the presence of validated biomarkers, including IDH mutations.
IHC is an affordable and simple procedure that can be performed with few resources, a powerful technique to study localization and presence/absence of a target at the tissue and cellular level, paraffin embedded and frozen tissue samples can be stored and accessed when required, and stained tissue sections can be stored and referred to whenever required. However, the specificity of antibodies can be variable and needs to be thoroughly checked using appropriate controls. The method is semi-quantitative, and the absolute abundance of the target cannot be reliably determined; the tissue is highly processed and may lead to a loss of information about the natural state. IHC is a multi-step procedure, and variability can be introduced at any stage, leading to poor reproducibility of results.[
Study limitation
Limitations of this study should be considered, such as the small sample size and lack of control for confounders, may be issued to the retrospective design study.
CONCLUSION
Assessing IDH1 status is critical for predicting survival rates and directing treatment decisions for patients with gliomas. Despite the lesser sensitivity and specificity of IHC in comparison to DNA sequencing in this study, larger prospective studies are needed to validate our preliminary finding.
Ethical approval
The research/study was approved by the Institutional Review Board at the Faculty of Medicine, Universitas Padjadjaran, Bandung, number 34/UN6.KEP/EC/2022, dated November 20, 2022.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
This study supported by the Grants-in-Aid from Indonesian Ministry of Education, Culture, Research and Technology, Grant No. 074/E5/PG.02.00.PL/2024 for Fundamental Research Grant.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Agnihotri S, Aldape KD, Zadeh G. Isocitrate dehydrogenase status and molecular subclasses of glioma and glioblastoma. Neurosurg Focus. 2014. 37: E13
2. Bolly HM, Faried A, Hermanto Y, Lubis BP, Tjahjono FP, Hernowo BS. Analysis of mutant isocitrate dehydrogenase 1 immunoexpression, Ki-67 and programmed death ligand 1 in diffuse astrocytic tumours: Study of single center in Bandung, Indonesia. J Korean Neurosurg Soc. 2021. 64: 100-9
3. Capper D, Zentgraf H, Balss J, Hartmann C, von Deimling A. Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol. 2009. 118: 599-601
4. Chen N, Yu T, Gong J, Nie L, Chen X, Zhang M. IDH1/2 gene hotspot mutations in central nervous system tumours: Analysis of 922 Chinese patients. Pathology. 2016. 48: 675-83
5. Elkhaled A, Jalbert LE, Phillips JJ, Yoshihara HA, Parvataneni R, Srinivasan R. Magnetic resonance of 2-hydroxyglutarate in IDH1-mutated low-grade gliomas. Sci Transl Med. 2012. 4: 116ra5
6. Faried A, Bolly HM, Hermanto Y, Achmad A, Halim D, Tjahjono FP. Prognostic significance of L-type amino acid transported-1 (LAT-1) expression in human astrocytic gliomas. Interdiscip Neurosurg. 2021. 23: 100939
7. Hata N, Suzuki SO, Murata H, Hatae R, Akagi Y, Sangatsuda Y. Genetic analysis of a case of glioblastoma with oligodendroglial component arising during the progression of diffuse astrocytoma. Pathol Oncol Res. 2015. 21: 839-43
8. Howard TP, Vazquez F, Tsherniak A, Hong AL, Rinne M, Aguirre AJ. Functional genomic characterization of cancer genomes. Cold Spring Harb Symp Quant Biol. 2016. 81: 237-46
9. Huang LE. Friend or foe-IDH1 mutations in glioma 10 years on. Carcinogenesis. 2019. 40: 1299-307
10. Iurlaro R, León-Annicchiarico CL, Muñoz-Pinedo C. Regulation of cancer metabolism by oncogenes and tumor suppressors. Methods Enzymol. 2014. 542: 59-80
11. Kalkan R, Atli Eİ Özdemir M, Çiftçi E, Aydin HE, Artan S. IDH1 mutations is prognostic marker for primary glioblastoma multiforme but MGMT hypermethylation is not prognostic for primary glioblastoma multiforme. Gene. 2015. 554: 81-6
12. Lee JH, Lee JE, Kahng JY, Kim SH, Park JS, Yoon SJ. Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature. 2018. 560: 243-7
13. Liu Y, Lang F, Chou FJ, Zaghloul KA, Yang C. Isocitrate dehydrogenase mutations in glioma: Genetics, biochemistry, and clinical indications. Biomedicines. 2020. 8: 294
14. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, FigarellaBranger D. The 2021 WHO Classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021. 23: 1231-51
15. Louis DN, Wesseling P, Aldape K, Brat DJ, Capper D, Cree IA. cIMPACT-NOW update 6: new entity and diagnostic principle recommendations of the cIMPACT-Utrecht meeting on future CNS tumor classification and grading. Brain Pathol. 2020. 30: 844-56
16. Macaulay RJ. Impending impact of molecular pathology on classifying adult diffuse gliomas. Cancer Control. 2015. 22: 200-5
17. Mahmoud MS, Khalifa MK, Nageeb AM, Ezz El-Arab LR, El-Mahdy M, Ramadan A. Clinical impact of IDH1 mutations and MGMT methylation in adult glioblastoma. Egypt J Med Hum Genet. 2024. 25: 42
18. Mebratie DY, Dagnaw GG. Review of immunohistochemistry techniques: Applications, current status, and future perspectives. Semin Diagn Pathol. 2024. 41: 154-60
19. Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013. 19: 764-72
20. O’Hurley G, Sjöstedt E, Rahman A, Li B, Kampf C, Pontén F. Garbage in, garbage out: A critical evaluation of strategies used for validation of IHC biomarkers. Mol Oncol. 2014. 8: 783-98
21. Picca A, Berzero G, Di Stefano AL, Sanson M. The clinical use of IDH1 and IDH2 mutations in gliomas. Expert Rev Mol Diagn. 2018. 18: 1041-51
22. Polívka J, Pešta M, Pitule P, Hes O, Holubec L, Polívka J. IDH1 mutation is associated with lower expression of VEGF but not microvessel formation in glioblastoma multiforme. Oncotarget. 2018. 9: 16462-76
23. Reuss DE, Sahm F, Schrimpf D, Wiestler B, Capper D, Koelsche C. ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an “integrated” diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta Neuropathol. 2015. 129: 133-46
24. Sporikova Z, Slavkovsky R, Tuckova L, Kalita O, Megova HM, Jiri E. IDH1/2 mutations in patients with diffuse gliomas: A single center retrospective massively parallel sequencing analysis. Appl Immunohistochem Mol Morphol. 2022. 30: 178-83
25. Tabei Y, Kobayashi K, Saito K. Survival in patients with glioblastoma at a first progression does not correlate with isocitrate dehydrogenase (IDH)1 gene mutation status. Jpn J Clin Oncol. 2021. 51: 45-53
26. Takahashi Y, Nakamura H, Makino K, Hide T, Muta D, Kamada H. Prognostic value of isocitrate dehydrogenase 1, O6-methylguanine-DNA methyltransferase promoter methylation, and 1p19q co-deletion in Japanese malignant glioma patients. World J Surg Oncol. 2013. 11: 284
27. Tateishi K, Yamamoto T, editors. IDH-mutant gliomas. London: Intech Open; 2019. p. 84543
28. Wen PY, Packer RJ. The 2021 WHO Classification of tumors of the central nervous system: Clinical implications. Neurooncology. 2021. 23: 1215-7
29. Zou Y, Bai HX, Wang Z, Yang L. Comparison of immunohistochemistry and DNA sequencing for the detection of IDH1 mutations in gliomas. Neuro Oncol. 2015. 17: 477-8