- Chief of Neurosurgical Spine and Education, Department of Neuro Science, Winthrop University Hospital, Mineola, NY 11501, USA
Correspondence Address:
Nancy E. Epstein
Chief of Neurosurgical Spine and Education, Department of Neuro Science, Winthrop University Hospital, Mineola, NY 11501, USA
DOI:10.4103/2152-7806.156559
Copyright: © 2015 Epstein NE. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.How to cite this article: Epstein NE. Preliminary documentation of the comparable efficacy of vitoss versus NanOss bioactive as bone graft expanders for posterior cervical fusion. Surg Neurol Int 07-May-2015;6:
How to cite this URL: Epstein NE. Preliminary documentation of the comparable efficacy of vitoss versus NanOss bioactive as bone graft expanders for posterior cervical fusion. Surg Neurol Int 07-May-2015;6:. Available from: http://surgicalneurologyint.com/surgicalint_articles/preliminary-documentation-comparable-efficacy-vitoss/
Abstract
Background:Laminectomies with posterior cervical instrumented fusions often utilize bone graft expanders to supplement cervical lamina/iliac crest autograft/bone marrow aspirate (BMA). Here we compared posterior fusion rates utilizing two graft expanders; Vitoss (Orthovita, Malvern, PA, USA) vs. NanOss Bioactive (Regeneration Technologies Corporation [RTI: Alachua, FL, USA]).
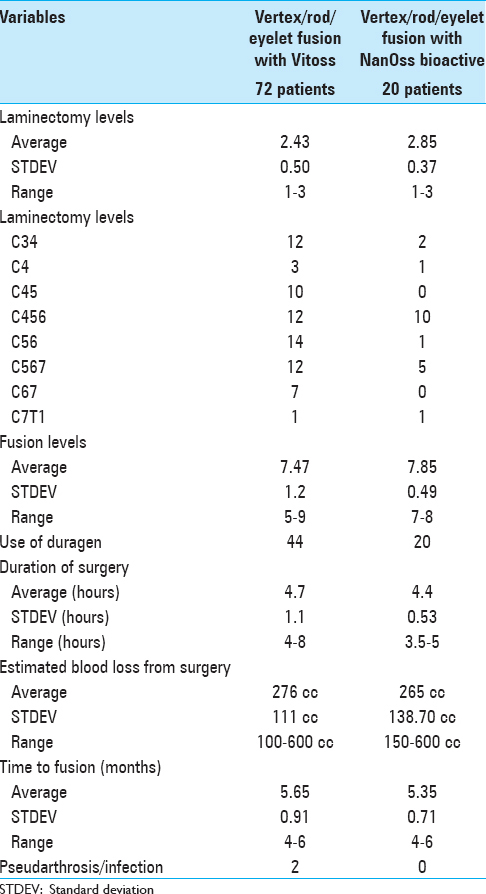
Methods:Two successive prospective cohorts of patients underwent 1-3 level laminectomies with 5-9 level posterior cervical fusions to address cervical spondylotic myelopathy (CSM) and/or ossification of the posterior longitudinal ligament (OPLL). The first cohort of 72 patients received Vitoss, while the second cohort or 20 patients received NanOss. Fusions were performed utilizing the Vertex/Rod/Eyelet System (Medtronic, Memphis, TN, USA) with braided titanium cables through the base of intact spinous processes (not lateral mass screws) cephalad and caudad to laminectomy defects. Fusion was documented by an independent neuroradiologist blinded to the study design, utilizing dynamic X-rays and two dimensional computed tomography (2D-CT) studies up to 6 months postoperatively, or until fusion or pseudarthrosis was confirmed at 1 year.
Results:Vitoss and NanOss resulted in comparable times to fusion: 5.65 vs. 5.35 months. Dynamic X-ray and CT-documented pseudarthrosis developed in 2 of 72 Vitoss patients at one postoperative year (e.g. bone graft resorbed secondary to early deep wound infections), while none occurred in the 20 patients receiving NanOss.
Conclusion:In this preliminary study combining cervical laminectomy/fusions, the time to fusion (5.65 vs. 5.35 months), pseudarthrosis (2.7% vs. 0%), and infection rates (2.7% vs. 0%) were nearly comparable sequentially utilizing Vitoss (72 patients) vs. NanOss (20 patients) as bone graft expanders.
Keywords: Autograft, beta-tricalcium phosphate, fusion/pseudarthrosis rates, instrumented fusion, NanOss bioactive, posterior cervical surgery, Vitoss
INTRODUCTION
Two successive prospective cohorts of patients underwent 1-3 level cervical laminectomies with 5-9 level posterior cervical fusions to address cervical spondylotic myelopathy (CSM) and/or ossification of the posterior longitudinal ligament (OPLL) [Figures
Figure 1
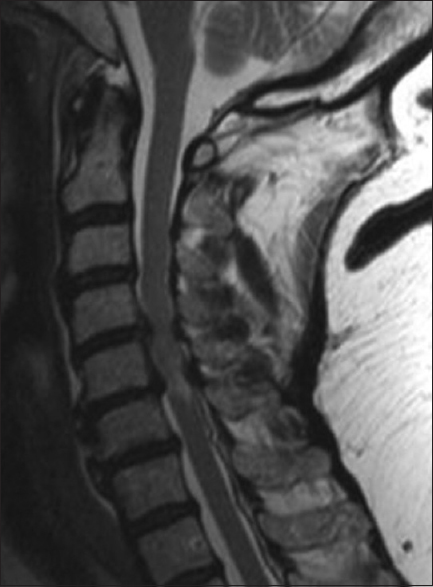
This classical midline sagittal T2-weighted MR study showed a marked hyperintense signal in the cervical cord opposite the C5-C6 level and multilevel ventral and dorsolateral compression (shingling of laminae/ossification of the yellow ligament) particularly involving the C4-C5 and C5-C6 levels. This patient successfully underwent a laminectomy of C4, C5, C6 with posterior fusion C2-T2
Figure 2
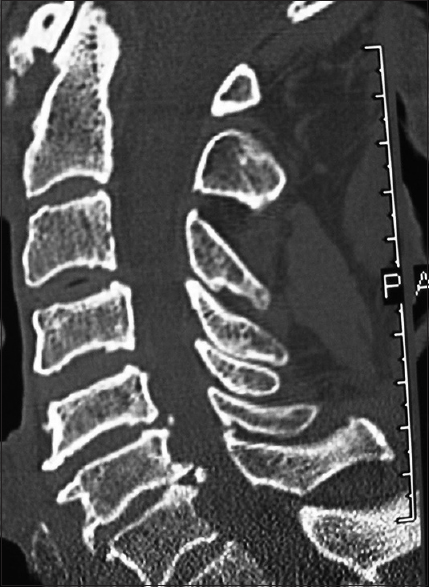
This classical midline sagittal 2D-CT study documents marked spinal stenosis with CSM accompanied by ventral OPLL (segmental behind the vertebral bodies of C4, C5, C6 with punctate ossification) and marked dorsolateral laminar shingling (e.g., C4, C5, C6 and the leading edge of C7). This patient's myelopathy resolved following a laminectomy of C4-C6 with undercutting of C3 and C7 and posterior C2-T2 fusion
Figure 3
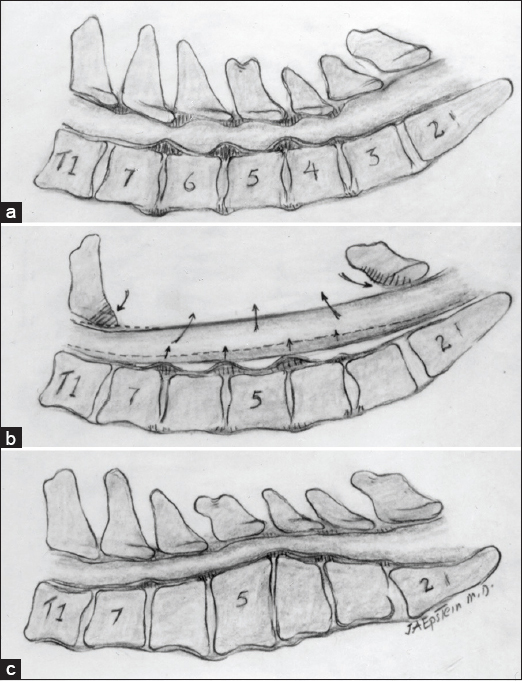
(a) A midline sagittal illustration of multilevel C3-C7 CSM accompanied by both ventral and dorso-lateral compression. (b) Cervical laminectomy C3-C7 for multilevel CSM in the presence of lordosis allows for dorsal cord migration away from ventrally situated osteophytes/pathology.(c) A laminectomy is contraindicated with kyphosis as the cord will fail to migrate posteriorly away from marked ventral compression
Figure 4
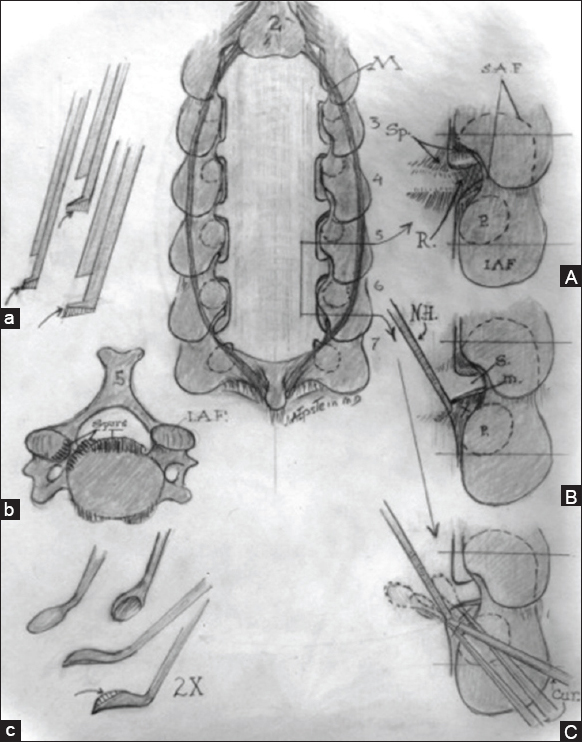
Midline Image: Cervical laminectomy C3-C7 is illustrated with medial facetectomy/foraminotomy performed at each intervening level. (a) Filed-down Kerrison punches for posterior cervical surgery. (b). Axial image of ventral cervical osteophyte (c). Down-biting curettes utilized for postero-lateral spur excision (A). Medial facetectomy/foraminotomy for exposure of the nerve root exiting at each level. (B). Use of a nerve hook to dissect/ gently retract the nerve root cephalad/medially in preparation for resection of underlying spur. (C). Use of down-biting curette to remove ventral spur
MATERIALS AND METHODS
Vitoss
Vitoss, a form of beta tri-calcium phosphate (B-TCP), is synthetic cancellous bone graft substitute/bone void filler that is comprised of 39% calcium and 20% phosphorous, in a 1:5 ratio.[
NanOss bioactive
NanOss’ nano-crystalline conformation (15–100 nm) mimics normal human bone crystals (25–500 nm) along with bone's composition/shape, while other calcium phosphate crystals are typically 1000–10,000 nm in size.[
Cervical laminectomy and posterior cervical fusions sequentially utilizing two bone graft supplements
In this series, one surgeon performed 92 consecutive 1-3 level laminectomies with posterior Vertex/Rod/Eyelet fusions (5-9 levels). These procedures utilized titanium cables placed through intact cephalad/caudad spinous processes, but no lateral mass screws (e.g. not Food and Drug Administration approved at the time) [
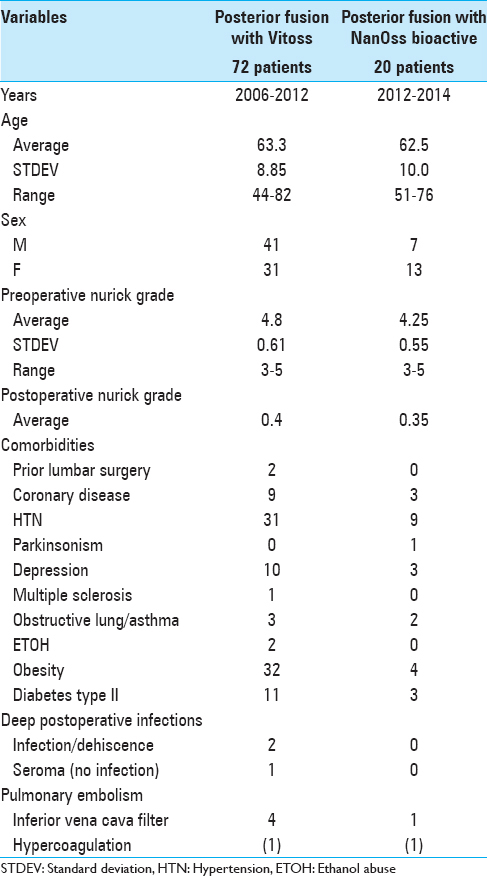
Similar clinical parameters for the patient populations receiving vitoss vs. NanOss
Clinical parameters were similar for both patient populations undergoing cervical laminectomies/posterior cervical fusions with Vitoss or NanOss to address multilevel CSM and/or OPLL documented on both magnetic resonance (MR) imaging and computed tomography (CT) studies [Figures
Surgery
One to three level laminectomies (e.g. including bilateral medial facetectomy/foraminotomies) were performed under an operating microscope utilizing diamond drills and small 1–2 mm Kerrison rongeurs to remove lateral bone. This was followed by 5-9 level (e.g. typical C2-T2) Vertex/Rod/Eyelet fusions performed utilizing braded titanium cables (no lateral mass screws) passed through the base of intact cephalad/caudad spinous processes.
Similar techniques for applying 2.5 × 10 cm strips of vitoss vs. Nanoss over residual/intact laminae/facets to complete the fusion
Each product comes in a 10 × 2.5 cm sheet, which is soaked in 10 cc of iliac crest BMA. Each sheet is then cut into ¼ longitudinal strips. Next, decortication is performed over the lateral aspect of the facets and residual laminae at the laminectomy site, followed by decortication of the cephalad/caudad intact laminae/facet joints. Next, very small (morcellated) cancellous iliac crest bone chips are applied laterally at the laminectomy site (over the residual laminae and facets); dorsal to the autograft, 1/4 to 1/8 inch longitudinal strips of Vitoss or NanOss are placed to supplement the bone graft but avoid dural impingement. Finally, cancellous and cortical bone chips are applied over the intact laminae/facet joints, and followed by application of the remaining 1/4-inch strips. The dura is then covered with Duragen, and a medium Hemovac drain is placed in the epidural compartment.
Dynamic X-rays and two dimensional computed tomography documentation of fusion
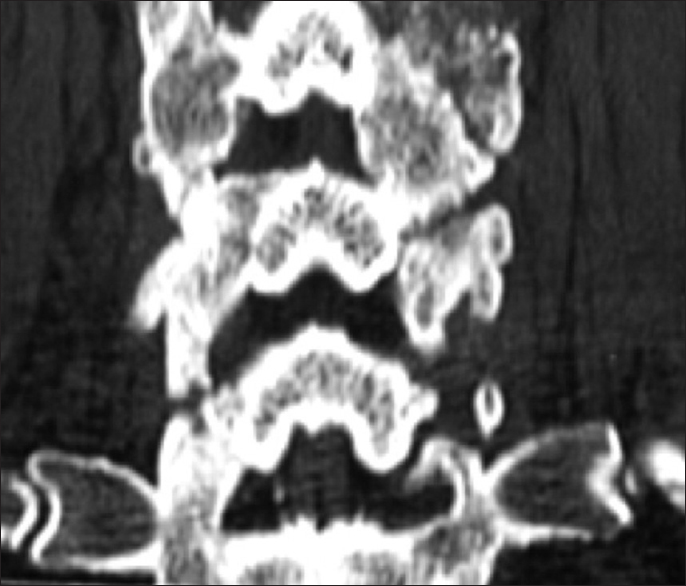
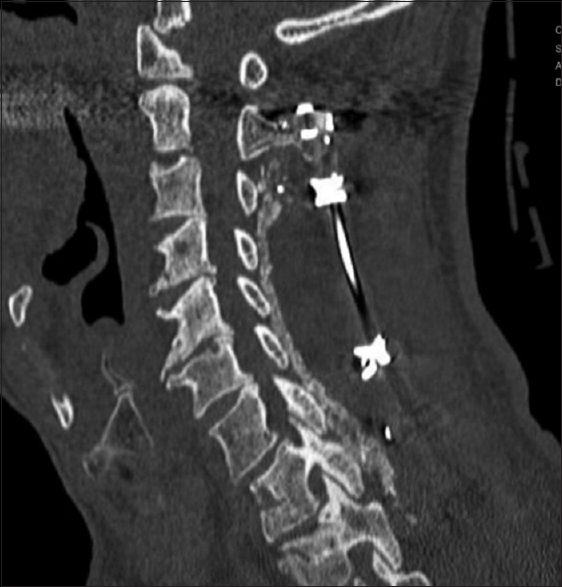
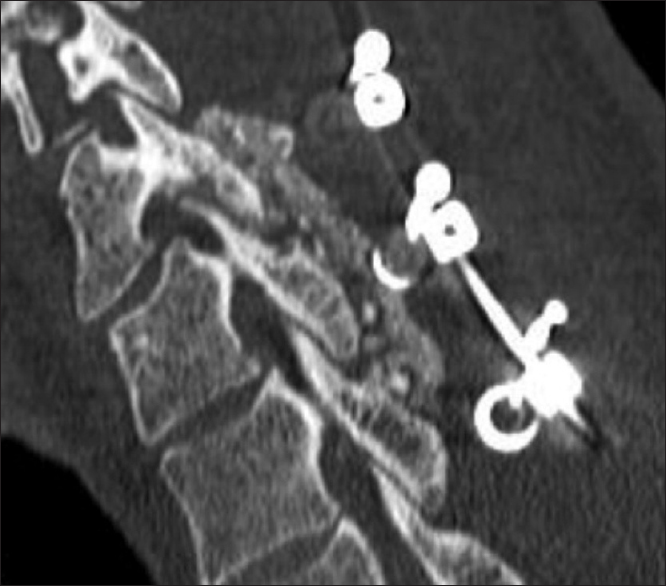
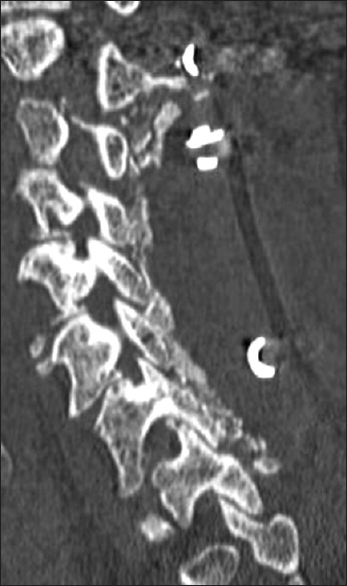
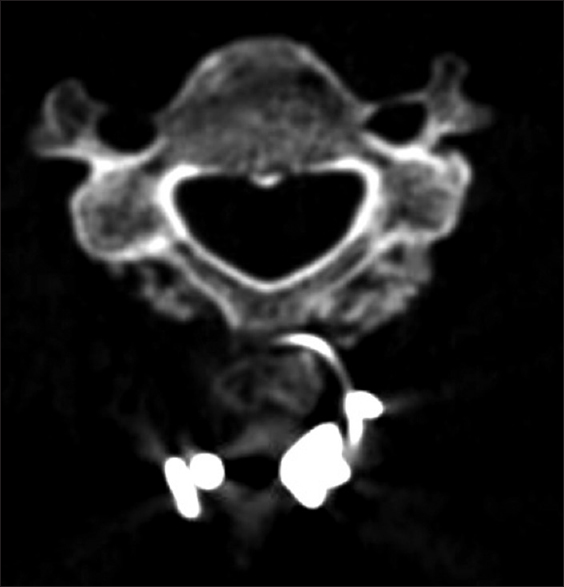
All patients underwent dynamic X-rays at 3, 4.5, and 6 months postoperatively (or until fused) along with two dimensional computed tomography (2D-CT) (e.g. performed at 3 postoperative months and repeated later if needed) [Figures
RESULTS
Fusion and pseudarthrosis rates
Patients in both the Vitoss and NanOss Groups demonstrated comparable times to fusion; 5.65 vs. 5.35 months postoperatively [Figures
No other complications directly related to either bone graft expander
No other complication observed in either group was uniquely attributable to Vitoss or NanOss [Tables
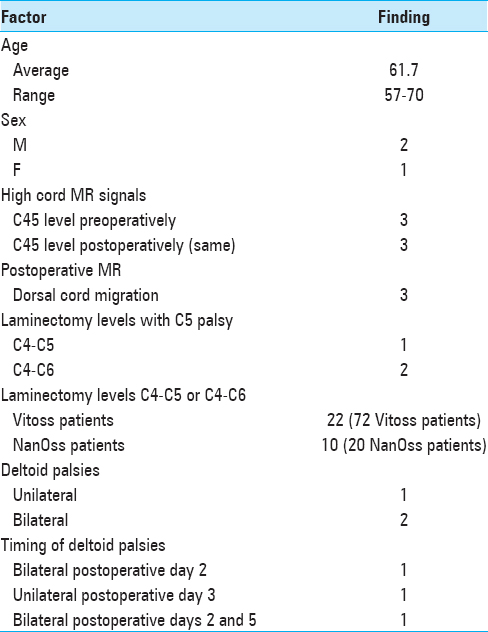
Three patients exhibiting delayed c5 root palsies
All procedures utilized intraoperative neural monitoring that included somatosensory-evoked potentials (SEP), electromyography (EMG), and motor-evoked potentials (MEP); no significant intraoperative changes occurred in any of the three cases where patients had delayed C5 palsies. Two C5 palsies occurred among the 72 receiving Vitoss, and one for the 20 patients receiving NanOss; none of these deficits were attributed to the bone graft expanders [
DISCUSSION
Data for vitoss bone graft expander for lumbar posterolateral fusions
Two series documenting instrumented posterolateral lumbar fusion rates utilizing vitoss
Epstein documented the frequency of successful instrumented posterolateral lumbar fusions (PLF) utilizing Vitoss to supplement autograft/BMA.[
Fusion rates utilizing vitoss for non-instrumented posterolateral lumbar fusion
In 2008, Epstein utilized Vitoss with lamina autograft and BMA to perform 60 multilevel laminectomies (average 5.8 levels) with 1- to 2-level noninstrumented PLF) in patients averaging 70 years of age.[
Level-1 pilot evaluation of vitoss/B-TCP as graft extender for posterior adolescent idiopathic scoliosis surgery
Lerner et al. compared the clinical/radiographic results for utilizing Vitoss/B-TCP vs. autogenous iliac crest bone graft (ICBG) in a prospective randomized scoliosis pilot study (EBM-Level 1).[
Bone morphogenetic protein and calcium phosphate salts for posterolateral lumbar fusion
Kaiser et al. proposed the use of local laminectomy autograft, calcium-phosphate salts, and bone morphogenetic proteins (BMPs) to supplement iliac autograft/local autograft for lumbar interbody fusions.[
NanOss: Nanocrystalline hydroxyapatite most comparable to normal bone vs. Vitoss
MacMillan et al. compared osteoblast and osteoclast activity for NanOss Bioactive (e.g. nanocrystalline hydroxyapatite [HA]; nanomaterials <100 nm; porous low crystalline nano-HA, B-TCP [RTI Surgical Corporation]) vs. other micron crystalline ceramics (e.g. calcium phosphate products; HA, and biphasic calcium phosphates [TCP/HA], porous micron-TCP [Vitoss; Stryker, Corporation, Kalamazoo MI, USA], various types of nanoceramics).[
A comparison of nanoss, autograft, and actifuse (baxter corporation franklin lakes, NJ, USA) in a rabbit posterolateral fusion model (nass meeting, 2009)
In 2009, Hill and Walsh (presentation North American Spine Society [NASS] Meeting 2009) observed that NanOss offers a high surface area for osteoblastic adhesions, proliferation, and bone mineralization in-vitro and in in-vivo animal models. They cited the surface areas of several different compounds: NanOss 70 m2/g, human bone 20–100 m2/g, Vitoss 0.3 m2/g, Actifuse 0.26 m2/g. While most collagen carriers utilize a triple helix structure, NanOss separates these strands providing more sites for cell infiltration/attachments and bone formation. They presented the data for PLF at the L5-L6 levels in rabbits and followed them with a combination of: Biomechanical testing, X-rays, CT, and histology. At 8 and 12 postoperative weeks, CT studies documented greater fusion for NanOss vs. Actifuse vs. Autograft, along with greater biomechanical strength/stiffness. Histology also revealed larger and more ossified/fused posterolateral fusion masses with NanOss vs. the other constructs. Again in 2012, Walsh et al. confirmed greater L5-L6 PLF in rabbits at 6, 12, and 26 weeks using similar testing parameters with comparable products (Orthopedic Research Society Meeting 2012) for NanOss with BMA/autograft vs. Vitoss BA/BMA vs. autograft/BMA.
Use of Nanocrystalline hydroxyapatite with autologous BMA and local bone in the lumbar spine. a retrospective CT of PLF
Robbins et al. performed a retrospective multicenter 1-year review of postoperative CT studies in 46 patients (average age 58.6) undergoing instrumented 1-3 segment PLF utilizing autograft, BMA, and NanOss.[
Comparable posterior cervical fusion rates utilizing vitoss vs. NanOss to supplement iliac crest bone graft and bone marrow aspirate
Posterior cervical instrumented fusion rates in this series were comparable for two sequential populations of patients undergoing 1-3 level cervical laminectomy with posterior instrumented fusions (5-9 levels). Fusion masses were comprised of lamina/iliac crest autograft (ICBG) and BMA supplemented with one of two bone graft expanders. The first cohort of 72 patients received Vitoss, while the second cohort of 20 patients received NanOss. The time to fusion for both groups, documented utilizing dynamic X-ray and 2D-CT studies were comparable (5.65 vs. 5.35 months) [
Contraindications for using NanOss
Contraindication to the use of NanOss include: Metabolic/systemic bone disorders negative impacting bone or wound healing, factures without stabilization, vascular impairment or graft site, infection (acute/chronic)/contamination, impaired calcium metabolism, steroid use, immunosuppression, use in open epiphyseal growth plates, and allergy to porcine collagen products.
References
1. Epstein NE. A preliminary study of the efficacy of Beta Tricalcium Phosphate as a bone expander for instrumented posterolateral lumbar fusions. J Spinal Disord Tech. 2006. 19: 424-9
2. Epstein NE. An analysis of noninstrumented posterolateral lumbar fusions performed in predominantly geriatric patients using lamina autograft and beta tricalcium phosphate. Spine J. 2008. 8: 882-7
3. Epstein NE. Beta tricalcium phosphate: Observation of use in 100 posterolateral lumbar instrumented fusions. Spine J. 2009. 9: 630-8
4. Kaiser MG, Groff MW, Watters WC, Ghogawala Z, Mummaneni PV, Dailey AT. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 16: Bone graft extenders and substitutes as an adjunct for lumbar fusion. J Neurosurg Spine. 2014. 21: 106-32
5. Lerner T, Bullmann V, Schulte TL, Schneider M, Liljenqvist U. A level-1 pilot study to evaluate of ultraporous beta-tricalcium phosphate as a graft extender in the posterior correction of adolescent idiopathic scoliosis. Eur Spine J. 2009. 18: 170-9
6. MacMillan AK, Lamberti FV, Moulton JN, Geilich BM, Webster TJ. Similar healthy osteoclast and osteoblast activity on nanocrystalline hydroxyapatite and nanoparticles of tri-calcium phosphate compared to natural bone. Int J Nanomedicine. 2014. 9: 5627-37
7. Robbins S, Lauryssen C, Songer MN. Use of Nanocrystalline Hydroxyapatite with Autologous BMA and Local Bone in the Lumbar Spine. A Retrospecrtive Ct Analysis of Posterolatearl Fusion Results. J Spinal Disord Tech. 2014. p.