- Department of Neurosurgery, Hospital Civil “Dr. Juan I. Menchaca”, Guadalajara, Jalisco, México
Correspondence Address:
Sergio Valente Esparza Gutiérrez
Department of Neurosurgery, Hospital Civil “Dr. Juan I. Menchaca”, Guadalajara, Jalisco, México
DOI:10.4103/sni.sni_361_18
Copyright: © 2019 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Adrián Santana Ramírez, Pedro Ávila Rodríguez, Sergio Valente Esparza Gutiérrez, Oscar Gutiérrez Ávila. Preliminary experience in the management of brain and skull-base tumors with microwave ablation; feasibility guided by ultrasound, report from 23 cases. 08-Feb-2019;10:17
How to cite this URL: Adrián Santana Ramírez, Pedro Ávila Rodríguez, Sergio Valente Esparza Gutiérrez, Oscar Gutiérrez Ávila. Preliminary experience in the management of brain and skull-base tumors with microwave ablation; feasibility guided by ultrasound, report from 23 cases. 08-Feb-2019;10:17. Available from: http://surgicalneurologyint.com/surgicalint-articles/9201/
Abstract
Background:In surgery involving brain tumors, the use of new tools or equipment that allows for better results and improvement in the quality of life of the patients is mandatory. Microwave ablation (MWA) is a technique that has been used effectively since 1994 in the management of different kinds of tumors. The authors present their surgical experience with 23 cases of brain and skull-base tumors using MWA technique.
Methods:In all, 23 cases diagnosed with brain and skull-base tumors are described; all of these were treated with MWA as unique technique as a complement to conventional microsurgical tumor resection. In all cases, ultrasound imaging guidance was used. A thin antenna (caliber 14.5; MedWaves) was positioned through ultrasound images to a central intratumoral area, and then energy was applied for 1–3 min until the temperature sensor in the proximal position of the antenna reached 80–100°C. Through transoperative Doppler ultrasound images and surgical microscopy, changes in the generated ablation were observed. The said ablation led to a decrease in intratumoral blood flow, and the adjacent vascular and cerebral structures were preserved.
Results:The application of MWA during brain surgery was regarded as safe in all cases, as no permanent additional neurological deficit was detected. Intratumoral vascular flow was also reduced and tumor resection was facilitated. Likewise, a reduction in tumor volume was noted, and in others in whom the ablation was applied as a single therapy, a progressive destruction of the tumor was observed.
Conclusion:MWA can be a useful tool as a single therapy or as a complement to conventional techniques for the surgical resection of brain and skull-base tumors. It was a safe method in all cases, producing a decrease in intratumoral blood flow, and this procedure facilitates the microsurgical resection of the lesion.
Keywords: Brain tumor, glioblastoma, microwave ablations, ultrasound imaging guide
BACKGROUND
Brain tumor surgery requires good planning and specific tools, such as an ultrasonic aspirator, bipolar instruments, and microsurgery instruments, among others. The use of new tools or equipment, which allows better results and improvement in the quality of life, is mandatory. Technological innovation is an important factor in the development of new surgical procedures and techniques.
Microwave ablation (MWA) is a technique that has been used with good results since 1994 in liver and pancreatic tumors. MWA has also been used to treat tumors of the bone, bladder, prostate, and kidney, and even to treat cardiac arrhythmias. However, the present work would appear to detail the first cases of brain and skull-base tumors treated with this particular technique.
In this study, we present the experience with this type of therapy in 23 patients with brain and skull-base tumors. We show the related technical aspects, management implications, postoperative results, subsequent follow-up, and also a review of the medical literature.
METHODS
A clinical study
This study is a 4-year observational, prospective inquiry.
The following were proposed as inclusion criteria for the patients to be treated:
- Patients with brain tumors and tumors of the skull that are not found in eloquent areas (the brainstem, areas: the senses, motor, language, vision, etc.)
- Patients with this type of lesion that has a tumor no less than 5.5 cm away from an eloquent area
- Patients with at least two failed attempts at surgical resections of lesions that meet the previous criteria
- Patients who have been explained all the different management alternatives available and to whom MWA has been proposed with its potential and respective risks. In the context of being part of a clinical study, the patients accepted treatment with this technique.
As exclusion criteria, the following was proposed:
- Patients with tumors of the central nervous system and skull base that are found in eloquent areas (the brainstem, areas: the senses, motor, language, vision, etc.).
- Patients with this type of lesion that has a tumor less than 5.5 cm away from an eloquent area.
- Patients who have been explained all the different management alternatives available and to whom MWA has been proposed with its potential and respective risks; in the context of being part of a clinical study, the patients did not accept treatment with this technique.
- Pregnant women.
- Patients with pacemakers or other implanted electromagnetic devices.
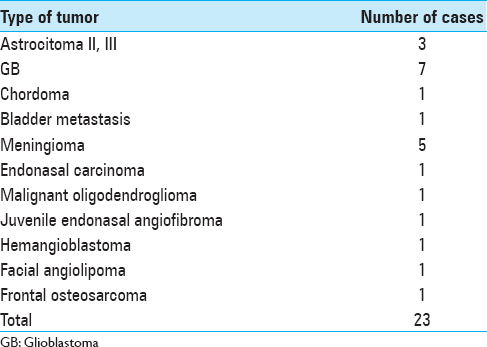
A total of 23 patients with a wide histological variety of brain and skull-base tumors, with an age range from 13 to 83 years (mean, 51 years) and lesions ranging from astrocytomas, glioblastomas, chordoma, bladder metastasis, meningiomas, endonasal carcinoma, hemangioblastoma, nasopharyngeal angiofibroma, oligodendroglioma, frontal osteosarcoma to facial angiolipoma, were included [
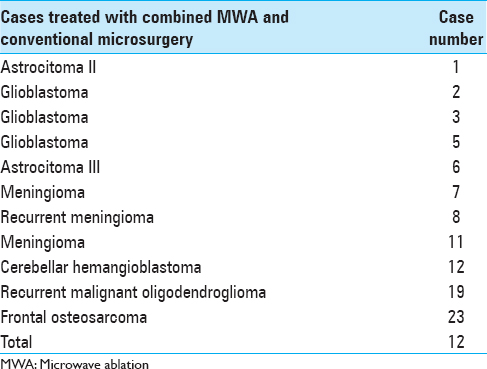
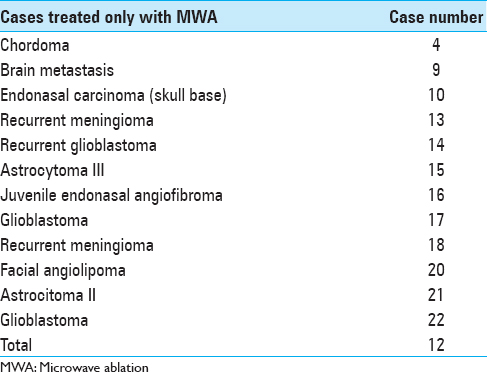
A total of 23 lesions were treated with ultrasound-guided MWA. In 11 of the patients treated with MWA, the treatment was performed in conjunction with conventional microsurgery, and in the other 12 cases, only MWA was applied in one or two sessions/interventions [Tables
All patients received a broad explanation of their treatment options including surgical resection, radiosurgery, conventional radiation, and chemotherapy including the technique of MWA in the context that said technique is part of a study protocol approved by the Institutional Review Board. The patients accepted the treatment offered and signed informed consent forms.
The follow-up period varied from 4 months in one patient (because it is the most recent case) up to 4 years in others.
Surgical procedure
All patients were premedicated with dexamethasone, saline, antibiotics, and light sedation; a general anesthetic was applied, and vital sign monitoring was obtained.
In most cases, a small craniotomy 2–3 cm in diameter was performed to access the tumor area, as well as to allow simultaneous use of the ultrasound transducer on the dural and/or cerebral cortex surface.
Epidural and transcortical sonographic visualization was performed in all patients with brain tumors. This visualization was performed to determine the volume, depth, tumor circulation, and adjacent cerebral structures by means of Doppler effect.
A durotomy of approximately 10 mm was performed with a biopsy directed at the tumor. An ultrasound-guided biopsy was also completed in all cases to determine the histological diagnosis.
The ablation antenna was directed toward the tumor using ultrasound-guided techniques to gain access to the central portion of the lesion. The antenna allowed visualization in real time of the vascular structures and individual tumor vessels so as to prevent bleeding and damage to adjacent structures. The antenna was repositioned from two to three vectors in all cases to cover the total volume of the tumor and to obtain ultrasonographic visualization every time the ablation was applied. Finally, visualization or final screening was performed by ultrasound with the aim of searching for the absence of bleeding or lesions in adjacent structures. The incision was closed in a normal conventional manner.
Microwave ablation
MWA was performed and directly viewed at all times by transoperative ultrasound through a small craniotomy for epidural or cortical placement of the transducer. The procedure occurred in such a way that it allowed the guided introduction of the probe verifying the exact location of the tip of the antenna vis-à-vis its orientation toward the center of the tumor. The procedure also allowed for visualization through a surgical microscope.
The effects of the treatment were evaluated clinically and by magnetic resonance image in the first, third, and sixth month and then every 5 or 6 months after the ablation. In some cases, this evaluation and follow-up continued for 4 years.
In all cases, the tumor was located with an imaging guide through the use of Aloka 5500 transoperative ultrasound. A thin antenna (caliber 14.5; MedWaves) was inserted in the central portion of the tumor. Energy was applied for 3–9 min with 45 W of power and in two or three different vectors until the temperature sensor in the antenna reached 80–100°C. During the ablation process, the recorded temperature of the cortical brain surface was 33–36°C. To record this measurement of the temperature, an electronic thermometer (sterile esophageal thermometer) was included and used in the ablation protocol. With a temperature sensor on its tip, the thermometer was placed in the cortical area adjacent to the tumor. We decided to use this device (sterile esophageal thermometer) because it is easy to manipulate and place. It is not invasive, offers constant temperature control, and does not interfere with other electromagnetic devices. Moreover, we did not have other devices in our institution capable of taking measurements of the temperature at deeper levels or at the point of interface of the tumor and brain tissue. The cortical cerebral surface was maintained with a constant soaking of 0.9% saline solution as a supplement to dissipate the heat.
Ablation changes were observed in real time, as well as the preservation of the adjacent cerebral and vascular structures, by means of a surgical microscope and transoperative ultrasound.
RESULTS
Clinical results
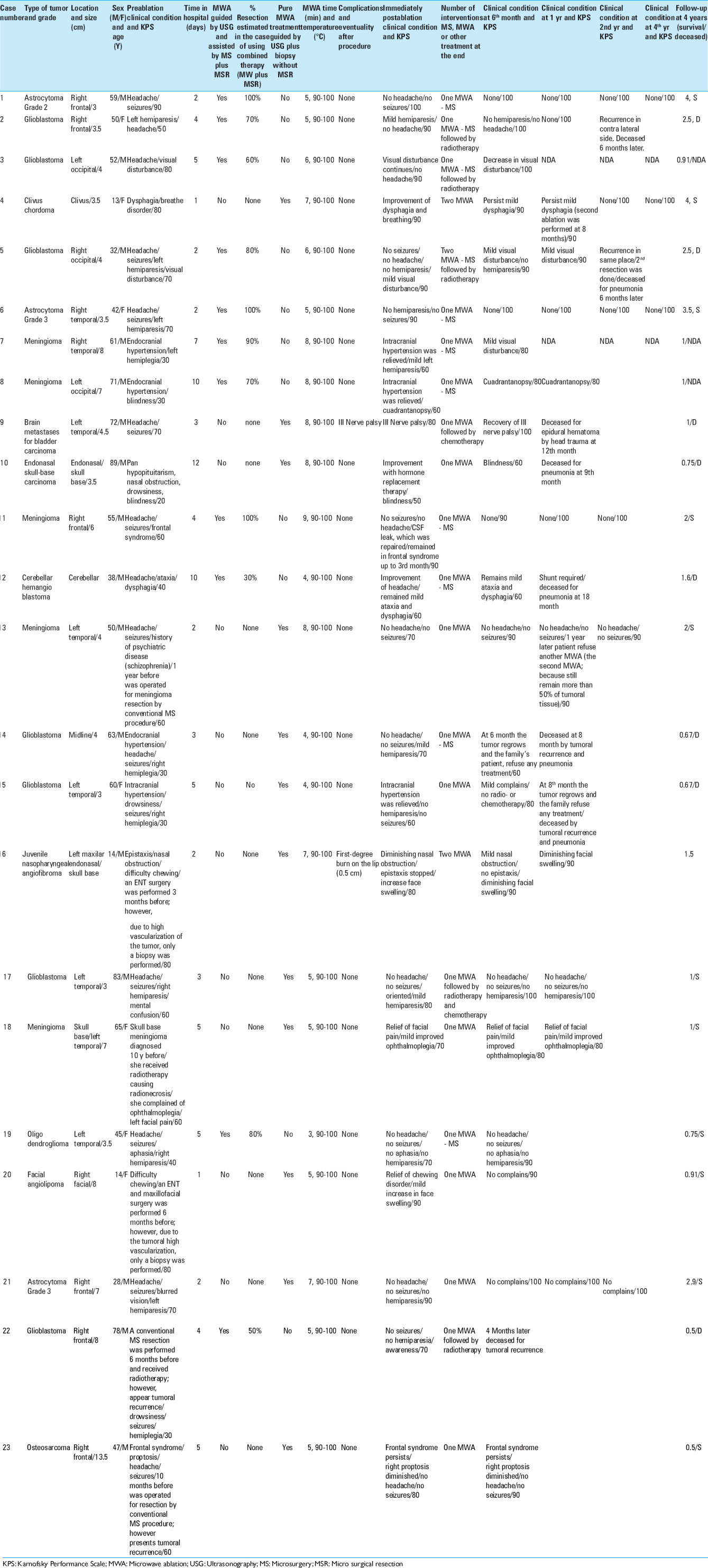
In our experience with the use of MWA as a complement to conventional microsurgery, the percentage of resection of the lesions that we achieved was 75.4%. In the postoperative and follow-up period, we achieved an improvement in the rating on the Karnofsky scale in 22 of the 23 cases.
Of the 23 patients treated, only 2 of them presented complications. These difficulties were transitory and did not lead to any deficit or permanent consequences. The first with complications was a 72-year-old patient with a left temporal tumor. In this individual, paralysis of the third ipsilateral cranial nerve occurred in the postoperative period, but this condition was self-limiting and dissipated within 48 h. The second patient was a 14-year-old adolescent with a nasoangiofibroma who developed a first-degree burn of 0.5 cm in the labial region. However, the said burn healed well and there was a satisfactory outcome.
We now present three representative cases of the utility of microwaves in which only biopsies are performed and which involve direct application of the antenna for intratumoral MWA.
Case 4
This case involves a 13-year-old girl who had a nasal obstruction and difficulty swallowing secondary to a chordoma of a growing clivus. She had experienced two failed attempts to remove the tumor. MWA was applied directly to the tumor under general anesthesia through a transoral approach using fluoroscopy to guide the ablation probe to the desired spot.
Symptoms improved immediately. Magnetic control resonance was realized and an appreciable diminution of tumor volume occurred. The application of a second session of 8 months followed achieving complete destruction of the tumor 1 year later. MRI images show the evolution of the lesion after two sessions of ablation [
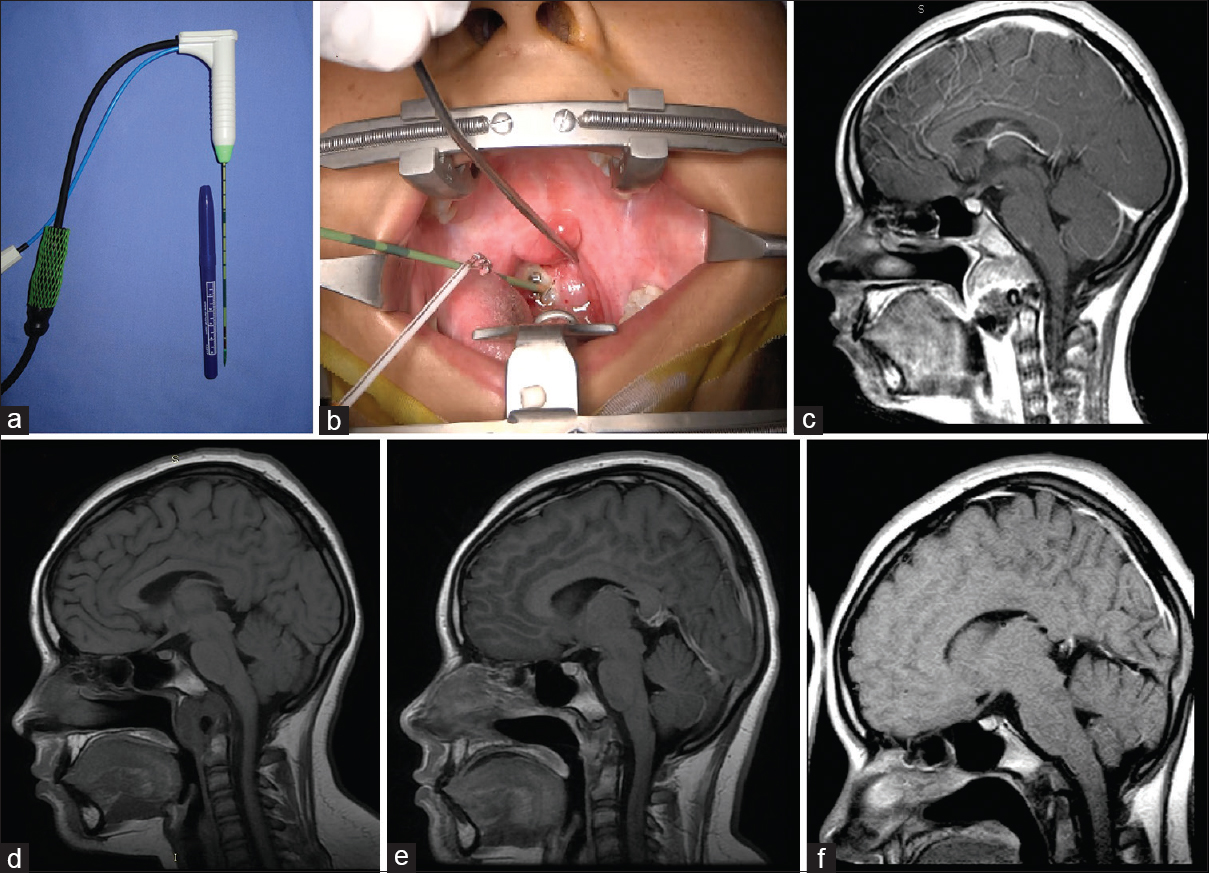
Figure 1
Case 4: (a) a thin antenna (14.5 gauge; MedWaves). (b) Transoral approach where the insertion of the ablation antenna toward the lesion is noted. (c) Magnetic resonance image enhanced in sagittal T1 (with contrast) where a clivus preablation lesion can be observed. (d) Magnetic resonance image enhanced in sagittal T1 8 months after ablation. (e) Magnetic resonance sagittal T1-enhanced image 14 months after ablation. (f) Sagittal magnetic resonance image enhanced in T1 30 months after ablation
Case 9
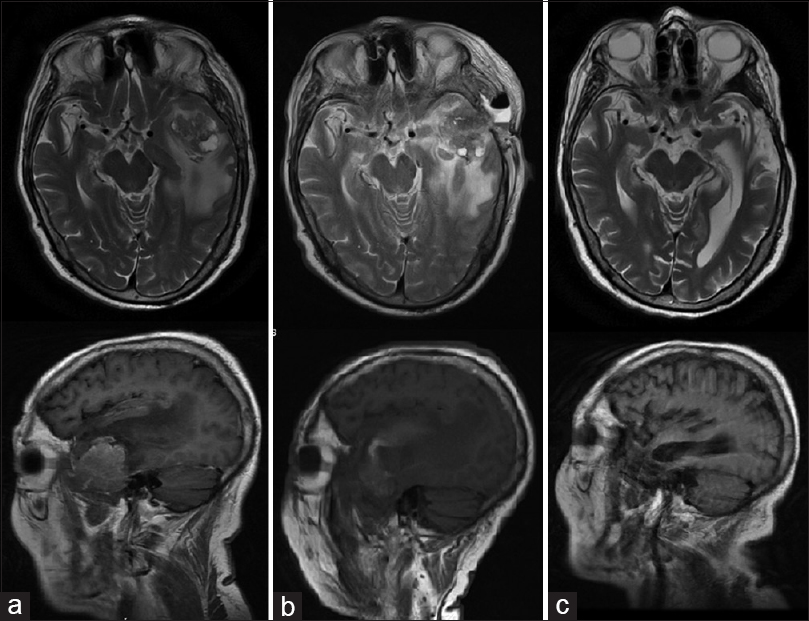
This case involves a male patient 72 years of age, with a recurrence of a tumor in the left temporal region (metastatic bladder carcinoma confirmed by histopathology); he was treated by another surgeon 4 months prior. The patient did not accept another conventional surgery or radiation therapy, so MWA was proposed.
A biopsy of the lesion was performed. Using ultrasound navigation, the tumor was accessed by placing the antenna in three different positions or vectors, trying to cover the entire volume of the lesion. After the first ablation, an immediate clinical improvement was seen. The patient was discharged from the hospital 3 days later. Time lapse MRI images show how the volume of the tumor decreases until its complete disappearance [
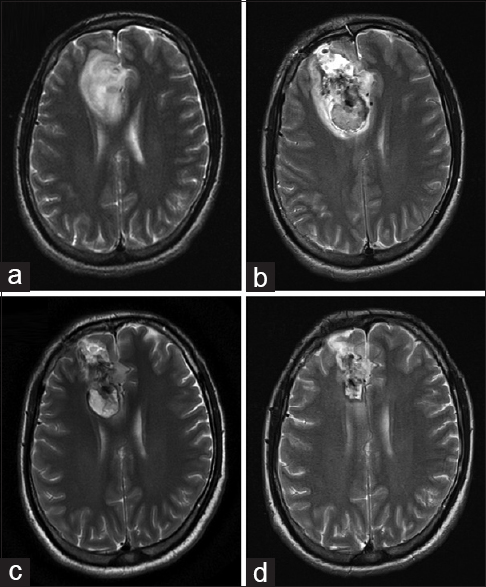
Case 21
A 27-year-old male patient had clinical data indicating headache and seizures. Magnetic resonance showed an intra-axial lesion in the right frontal region. The patient refused conventional surgical treatment, hence MWA was proposed. The treatment was carried out through a supraorbital craniotomy. Using a transoperative ultrasound, a biopsy was then performed. Ablation of the lesion was completed in three different vectors, ensuring the placement of the antenna in the intratumoral space. The patient was discharged from the hospital in good condition on the third day.
DISCUSSION
In the past 70 years, the application of microwaves in medicine and its most sustained clinical interest has been in the field of external heat therapy for the treatment of oncological diseases. Other areas of interest have emerged as additional viable fields of application are discovered. Many of these applications have been in the area of minimally invasive surgery, while others have application in wider areas of medical interest and especially surgery in general.[
The term ablation refers to the direct application of chemical or thermal therapies to a specific organ or tissue in an attempt to achieve substantial tissue destruction or eradication. The methods of tumor ablation most commonly used in current practice fall into two main categories, namely, chemical ablation and thermal ablation. Chemical ablation includes agents such as ethanol and acetic acid that induce necrosis by coagulation and tumor ablation per the case in question. Thermal ablation involves quite distinct techniques that use various methods to destroy tumors. These methods include ultrasound, radiofrequency, microwaves, laser energy, and cold cryoablation.[
In this clinical study, the type of thermal ablation consists of a microwave generator that emits an electromagnetic wave through the exposed area and which is not isolated from the antenna. Through an open or percutaneous incision, the surgeon has access to the tumor through a CT scan or the ultrasound guide which can determine the exact location of the tumor. Electromagnetic microwaves stir the water molecules in the surrounding tissue and produce friction and heat. This process induces cell death through coagulation necrosis and eliminates (destroys) the tumor tissue. The goal of MWA is to destroy the entire tumor, respecting as much as possible the normal range of the surrounding parenchyma, avoiding lesions in the critical structures, and quickly creating a wider ablation area.[
The role of regional treatments guided by imaging of patients with tumors of different histopathology and location has been growing substantially since the beginning of the last decade. These therapies range from the techniques of percutaneous ablation to open ablation. In the beginning, their application was oriented to purely palliative purposes; however, all too often they have been used for curative purposes.[
The use of ablation-based therapies through the insertion of a percutaneous catheter in the tumor lesion has been used and accepted in liver tumors. This catheter creates changes in the intratumoral temperature; however, there are already reports that this treatment has been used in tumors of the thyroid, kidneys, bones, prostate, breast, adrenal glands, and pancreas.[
In this context, for example, Tabuse et al. have reported since 1985 on the use of microwaves as a tissue coagulant in hepatectomy surgery.[
Compared with traditional techniques, local thermal ablations and guided regional image treatments offer an advantage with respect to reducing morbidity and mortality. This benefit is at a lower cost compared with the ramifications associated with traditional surgical procedures.[
There are few reported cases in which some type of thermotherapy was applied in brain or skull-base tumors. An example of this case involved laser-induced interstitial thermotherapy using stereotactic guidance (although different in the type of ablation proposed in the current work). This instance used a minimally invasive method to produce thermal necrosis in tumor brain tissue of tumors of glial origin. This method is a safer one which combined with radio- and chemotherapy techniques could be useful with regard to handling these types of injuries.[
In this sense, thermal/ablative treatment is receiving more and more attention as an alternative to standard surgical therapies, especially for patients with contraindications or who reject traditional or open surgery approach.[
CONCLUSION
Ultrasound-guided MWA in brain and skull-based tumor surgery, either used to assist in microsurgery or for MWA only, turns out to be, in our humble experience, a minimally invasive, safe, and useful technique for the treatment of some types of injuries.
Although they are the first cases in which MWA was applied, the dose of thermal energy applied has been the least possible to avoid damage to normal adjacent tissue. Applying higher doses of energy could possibly offer better results; however, these practices should be considered for future studies.
In our experience, with respect to the management of brain lesions and injuries at the base of the skull through the use of this technique, it is worth mentioning that not all lesions showed the same reaction to thermal ablation. This fact could be observed in these lesions in the postoperative period and through the study of images involving an inflammatory process of the tissue in ablation. This fact was also secondary to the effects of the heat to which they were exposed, as well as to the reaction to tissue destruction in the areas directly subjected to ablation in the lesions produced by metastasis, chordomas, and meningiomas.
By taking into account the following points, we have no doubt that this tool could be used in a wider and more beneficial manner in the field of neurosurgery:
MWA is a useful tool as a complement to conventional brain tumor microsurgery techniques MWA was safe in all cases The ablation of the tumor in question contributes to the reduction of intratumoral vascular blood flow. This reduction allows and facilitates microsurgical resection MWA is the sole technique in the management of certain cases of brain tumors. It would appear to be effective as it achieves the destruction of the lesion.
Given the above, MWA can be a viable alternative for high-risk brain tumor resection surgery. It can turn a patient with a very poor prognosis with an “inoperable” tumor into a candidate for surgery.
The availability of MWA in a technical sense can provide the benefit of surgical removal of some tumors that are difficult or impossible to access with conventional craniotomy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Beland MD, Dupuy DE, Mayo-Smith WW. Percutaneous cryoablation of symptomatic extraabdominal metastatic disease: Preliminary results. AJR Am J Roentgenol. 2005. 184: 926-30
2. Callstrom MR, Atwell TD, Charboneau JW, Farrell MA, Goetz MP, Rubin J. Painful metastases involving bone: Percutaneous image-guided cryoablation—Prospective trial interim analysis. Radiology. 2006. 241: 572-80
3. Chiang J, Wang P, Brace CL. Computational modelling of microwave tumour ablations. Int J Hyperthermia. 2013. 29: 308-17
4. Gaynor SL, Byrd GD, Diodato MD, Ishii Y, Lee AM, Prasad SM. Microwave ablation for atrial fibrillation: Dose-response curves in the cardioplegia-arrested and beating heart. Ann Thorac Surg. 2006. 81: 72-6
5. Gervais DA, Arellano RS, Mueller P. Percutaneous ablation of kidney tumors in nonsurgical candidates. Oncology (Williston Park). 2005. 19: 6-11
6. Habash RW, Bansal R, Krewski D, Alhafid HT. Thermal therapy, Part III: Ablation techniques. Crit Rev Biomed Eng. 2007. 35: 37-121
7. Huang SM, Hsu IC, Wu CW, Lui WY. A modified dising of microwave tissue coagulator monopolar antenna for applications in laparoscopic hepatic surgery. Surg Laparosc Endosc. 1998. 8: 44-8
8. Jindal G, Friedman M, Locklin J, Wood BJ. Palliative radiofrequency ablation for recurrent prostate cancer. Cardiovasc Intervent Radiol. 2006. 29: 482-5
9. Keane MG, Bramis K, Pereira SP, Fusai GK. Systematic review of novel ablative methods in locally advanced pancreatic cancer. World J Gastroenterol. 2014. 20: 2267-78
10. Laeseke PF, Winter TC, Davis CL, Stevens KR, Johnson CD, Fronczak FJ. Postbiopsy bleeding in a porcine model: Reduction with radio-frequency ablation – Preliminary results. Radiology. 2003. 227: 493-9
11. Lantis JCII, Carr KL, Grabowy R, Connolly RJ, Schwaitzberg SD. Microwave applications in clinical medicine. Surg Endosc. 1998. 12: 170-6
12. Leonardi MA, Lumenta CB. Stereotactic Guided Laser-Induced Interstitial Thermotherapy (SLITT) in gliomas with intraoperative morphologic monitoring in an open MR: Clinical experience. Minim Invas Neurosurg. 2002. 45: 201-7
13. Liapi E, Geschwind JF. Transcatheter and ablative therapeutic approaches for solid malignancies. J Clin Oncol. 2007. 25: 978-86
14. Mayo-Smith WW, Dupuy DE. Adrenal neoplasms: CT-guided radiofrequency ablation – Preliminary results. Radiology. 2004. 231: 225-30
15. Seki T, Wakabayashi M, Nakagawa T, Itho T, Shiro T, Kunieda K. Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer. 1994. 74: 817-25
16. Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: Principles and applications. Radiographics. 2005. 25: S69-83
17. Susini T, Nori J, Olivieri S, Livi L, Bianchi S, Mangialavori G. Radiofrequency ablation for minimally invasive treatment of breast carcinoma: A pilot study in elderly inoperable patients. Gynecol Oncol. 2007. 104: 304-10
18. Tabuse K, Katsumi M. Application of a microwave tissue coagulator to hepatic surgery the hemostatic effects on spontaneous rupture of hepatoma and tumor necrosis. Nippon Geka Hokan. 1981. 50: 571-9
19. Tabuse K, Katsumi M, Kobayashi Y, Noguchi H, Egawa H, Aoyama O. Microwave surgery: Hepatectomy using a microwave tissue coagulator. World J Surg. 1985. 9: 136-43
20. Tse HF, Liao S, Siu CW, Yuan L, Nicholls J, Leung G. Determinants of lesion dimensions during transcatheter microwave ablation. Pace. 2009. 32: 201-8