- Department of Neurosurgery, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh.
Correspondence Address:
Md Rezaul Amin, Department of Neurosurgery, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh.
DOI:10.25259/SNI_250_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Md Rezaul Amin, KM Tarikul Islam, Moududul Haque. Preoperative planning of craniectomy and reconstruction using three–dimension-printed cranioplasty for treatment of calvarial lesion. Surg Neurol Int 12-Jul-2024;15:241
How to cite this URL: Md Rezaul Amin, KM Tarikul Islam, Moududul Haque. Preoperative planning of craniectomy and reconstruction using three–dimension-printed cranioplasty for treatment of calvarial lesion. Surg Neurol Int 12-Jul-2024;15:241. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12988
Abstract
Background: Common calvarial lesions include fibrous dysplasia (FD), intraosseous meningioma, osteoma, Langerhans cell histiocytosis (LCH), intraosseous hemangioma, dermoid and epidermoid cyst, and malignancy. Surgical removal with removal of the involved skull is the choice of treatment for these lesions. Previously, the skull defect was repaired using allograft, and alloplastic materials have been replaced with newer polyetheretherketone (PEEK) material, which is more resistant, biocompatible, and can be 3-dimension (3D)-printed. High-resolution 3D printing uses very fine extruders to put materials in fine layers to recreate patients’ anatomy authentically, which gives superior cosmetic outcomes. Our objectives were preoperative planning of craniectomy and reconstruction for calvarial lesions and reconstruction of skull defects using 3D-printed cranioplasty with PEEK materials.
Methods: In this series, we describe 11 cases in which skull lesions were removed and reconstructed in the same sitting using a 3D-printed PEEK implant designed preoperatively using high-resolution computer tomography. All the cases were done in the neurosurgery department of Bangabandhu Sheikh Mujib Medical University from 2021 to 2023. Patients were followed up for 6 months after surgery.
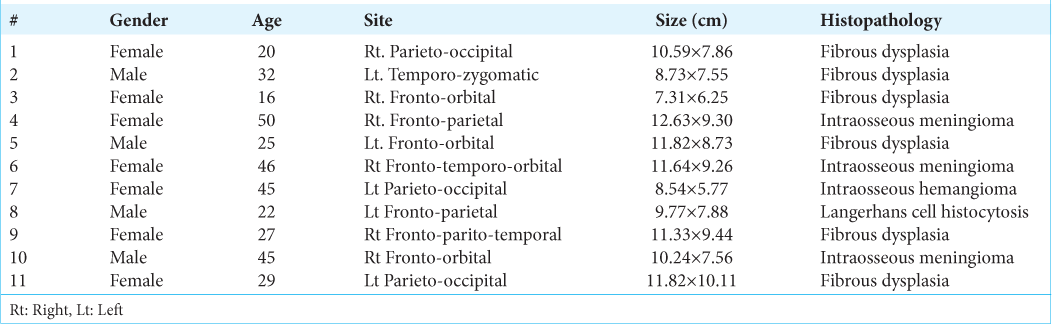
Results: Regarding 11 cases, six cases were FD, three cases were intraosseous meningioma, one case was intraosseous hemangioma, and one case was LCH. Average lesion size were 12.73-5.77 cm. Cranioplasty was done with PEEK material. Minor complications were treated conservatively. Seroma, postoperative fever, and nausea were among these.
Conclusion: The human bone-like biocompatibility and resistance to physical forces leads to more frequent use of PEEK, which enables to repair of complex craniofacial defects with better cosmesis. Despite some limitations, the PEEK cranioplasty implant continued to thrive and showed its promise to be an excellent material. Further, research and investment should be put into developing the technique.
Keywords: Calvaria, Cranioplasty, Fibrous dysplasia, Polyetheretherketone (PEEK)
INTRODUCTION
Complex calvarial lesions involving one or more regions of the skull are quite common. Many small lesions are osteomas or bony protuberances, which seldom pose any problem. However, the larger ones, which are noticeable from a distance and causing deformity, are also found. These lesions need removal and reconstruction cosmetically to ensure patient satisfaction. Larger lesions make big cranial defects, which, if not covered, may cause various neurological problems. Hence, cranioplasty must be done in these cases. Cranioplasty is probably the oldest neurosurgical procedure which is to repair cranial defects in both cosmetic and functional ways.[
Fibrous dysplasia (FD) is a disease where woven bone is replaced by dysplastic bone. It often affects craniofacial bone and causes obvious deformity and neurological deficit. Calvarial FD mainly causes deformity, rarely causing neurodeficit. Patients often need surgery to correct the disfigurement.[
With the advances in technology and manufacturing methods, different materials have been tried, but none could be deemed as perfect all-rounder candidates. In recent years, popular materials are polymethyl methacrylate (PMMA) bone cement, Titanium mesh or plates, and Polyetheretherketone (PEEK) which is an inert biocompatible polymer.[
In our series, we used PEEK implants, which were 3D-printed and single-stage orientation to replace, these implants after removal of skull lesions. In our country, due to expenses and technical difficulties, there are very limited uses for PEEK implants, which are 3D-printed. Hence, we want to set a guideline for using PEEK materials which are 3D-printed.
MATERIALS AND METHODS
A total of 11 cases are described here. All the cases were done in the Neurosurgery Department, Bangabandhu Sheikh Mujib Medical University, from 2021 to 2023. Our objectives were pre-operative planning of removal of lesions with involved skull bones and reconstruction of skull defects using 3D printed cranioplasty with PEEK materials. Multiple lesions, metastatic osteolytic lesions, and decompressive craniotomy for trauma or other causes were excluded from these series. Data were collected purposively. All the patients were presented with hard, bony swellings in the head. The deformity was obvious, and diagnoses were made using a computer tomography (CT) scan, magnetic resonance imaging (MRI), and other laboratory tests. The plan was to remove the lesion with the involved skull and repair the defect in the same operation. Patient recovery was monitored over the next week and followed up to 6 months. Complications were noted and treated if needed. A comparison with existing literature was done.
Technical details
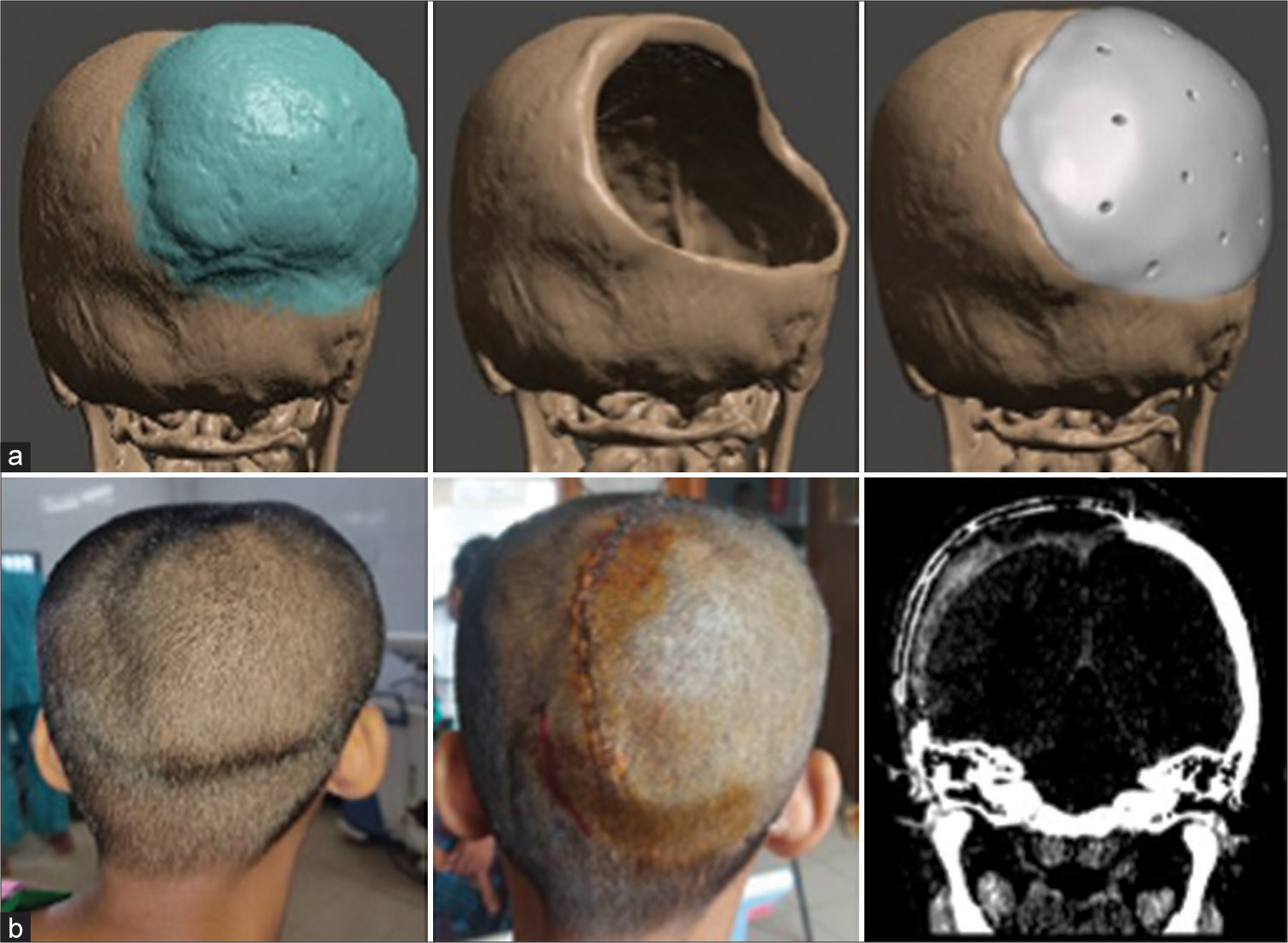
For preoperative planning, we did high resolution CT scan of the head of every patient. The latest multidetector spiral scanners provided up to 0.6mm interval slices, resulting in a very highly detailed 3D model. The scan data were retrieved as DICOM files and transferred to a computer workstation. 3D models were created using volume reconstruction tool protocol in CAD software. Initially, we used 3D slicer® software, which is an open source, free software supported by NIH. 11 Later, a more refined workflow was devised as we shifted to RadiAnt™ DICOM viewer to obtain the high-resolution 3D model from the DICOM data. The model was imported into another software, Meshmixer™.
A series of tools and algorithms were used to outline the lesion; then, a virtual craniotomy was planned. The craniotomy edges were defined and projected onto the opposite side using a mirror tool. Thus, the implant is designed with perfect edge fitting and contours from the patient’s own anatomy. Further, smoothening and sharpening are used to create aesthetically sound and accurate skull flaps.
The model is then sent to the manufacturer company for 3D printing. A high-resolution 3D printer prints the model using PEEK. Sometimes, a draft model is printed in PLA and sent to the surgeon for validation. After necessary revision and modification, the final surgeon-approved model is printed in PEEK. The implant is then sterilized in the central sterile services department by autoclaving and delivered in sterile packaging before operation [
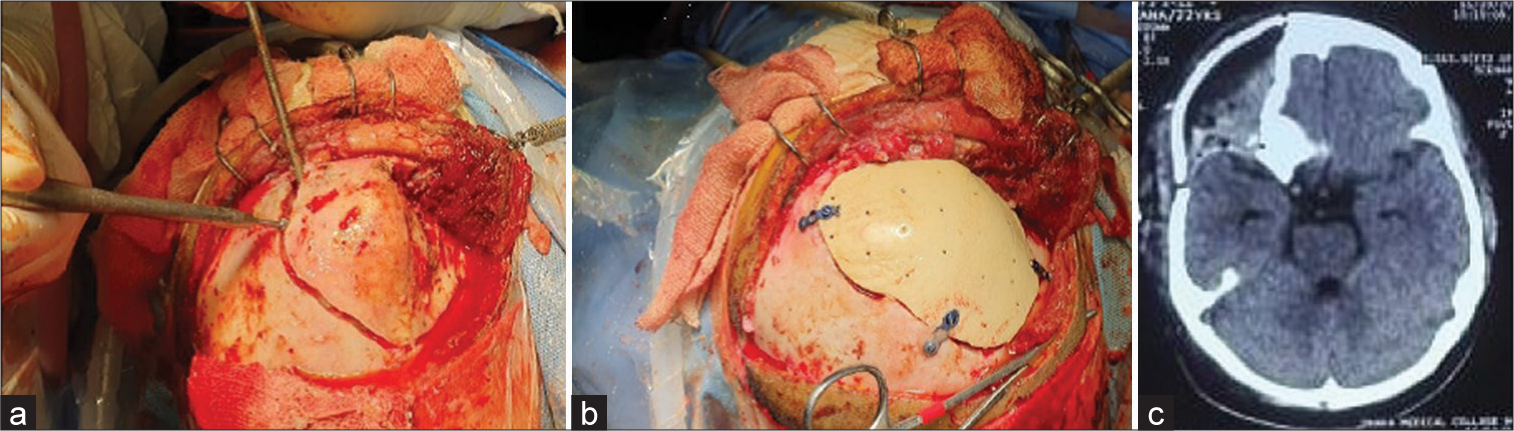
The operative steps were as usual. Every patient was counseled beforehand regarding the procedure. General anesthesia was given. Then, the patient was positioned according to the pathological site. Head fixed with a head frame. After sterile draping, a skin incision was given according to a planned line. Adequate exposure was made to make sure all the dysplastic bone margins were visible. Then, burr holes were made, and craniectomy was done using a high-speed craniotome according to the planned path. Removal of the lesion was done, and hemostasis was secured. Any dural sinus in the craniotomy margin was taken care of. Then, the prefabricated implant was placed in the defect and checked for fit. If any discrepancy were found and edge adjustment was done. Then, we used mini plates and screws to secure the implant onto the defect. Wound closure was done in the usual manner in multiple layers. A drain was kept in subgaleal space for 3 days. Skin closure was done using prolene sutures or skin staplers. The patient was observed in the recovery unit for some time. Postoperatively, we checked dressing and drain collection and took routine measures. CT scanning was done in the 1st week to check implant placement and fitting and to detect any complications. The patient was released after the removal of skin stitches [
RESULTS
Patient demographics and lesion parameters are shown in
One-month and 3 months follow-up schedules were instituted for every patient.
Minor complications were treated conservatively. Seroma 5 (45%), postoperative mild fever 3 (27%), and nausea were among these. All the patients had preoperative and postoperative Glasgow coma scale 15. Long-term complications such as implant infection, breakage, and resorption were not seen. There was no incidence of neurological deficit or seizure. We also tried to evaluate implant fit and cosmetic outcome arbitrarily and found them to be satisfactory.
DISCUSSION
In 3000 BC, archeological evidence suggests that cranial reconstruction after head trephination repair was done with metals, shells, and gourds. In subsequent years, the cranioplasty was done using moistened linen, which was applied over wounds, and regular dressings were performed to promote wound granulation.[
The reconstruction of cranial bone defects after resection of calvarial tumor has great importance for the protection of the brain’s vital structures and also for cosmetic purposes. The functional aspect of the procedure is the cosmetic restoration of the cranial contours.[
The choice of implant is dependent on various factors such as availability, surgeon’s preference, and patient discussion. Overall, liquid PMMA is the second most used material in cranioplasty behind titanium mesh. Both PEEK and solid PMMA CCIs are non-thermo-conductive and form stable.[
Using 3D printing and advanced biomaterials to repair cranial defects is a new concept, and this is the first case series of this technique in Bangladesh. In global literature, 3D-printed customized PEEK implants for cranioplasty are continually growing.[
Limitation of the study
Peek material is expensive in terms of our country Small size case no The study was conducted within a short period.
CONCLUSION
The human bone-like biocompatibility and resistance to physical forces lead to more frequent use of PEEK, which enables to repair of complex craniofacial defects with better cosmesis. Despite some limitations, the PEEK cranioplasty implant continues to thrive and shows its promise to be an excellent material. Further, research and investment should be put into developing the technique.
Ethical approval
The Institutional Review Board has waived the ethical approval for this study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Aciduman A, Belen D. The earliest document regarding the history of cranioplasty from the Ottoman era. Surg Neurol. 2007. 68: 349-53
2. Adetayo OA, Salcedo SE, Borad V, Richards SS, Workman AD, Ray AO. Fibrous dysplasia: An overview of disease process, indications for surgical management, and a case report. Eplasty. 2015. 15: e6
3. Coulter C, Richards R, Peterson D, Collier J. Parietal skull reconstruction using immediate peek cranioplasty following resection for craniofacial fibrous dysplasia. J Plast Reconstr Aesthet Surg. 2014. 67: e208-9
4. Colas L, Caron S, Cotton A. Skull vault lesions: A review. AJR Am J Roentgenol. 2015. 205: 840-7
5. Dodier P, Winter F, Auzinger T, Mistelbauer G, Frischer JM, Wang WT. Single-stage bone resection and cranioplastic reconstruction: Comparison of a novel software-derived PEEK workflow with the standard reconstructive method. Int J Oral Maxillofac Surg. 2020. 49: 1007
6. Dumbrigue HB, Arcuri MR, LaVelle WE, Ceynar KJ. Fabrication procedure for cranial prostheses. J Prosthet Dent. 1998. 79: 229-31
7. Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012. 30: 1323-41
8. Hosaain SM, editors. Comparison of surgical outcome of cranioplasty between 3D-printed polyetheretherketone (PEEK) patient specific implant and frozen autologous bone [Thesis]. Bangladesh: Bangabandhu Sheikh Mujib Medical University; January 2022. p.
9. Huang GJ, Zhong S, Susarla SM, Swanson EW, Huang J, Gordon CR. Craniofacial reconstruction with poly(methyl methacrylate) customized cranial implants. J Craniofac Surg. 2015. 26: 64-70
10. Kim JC, Hong IP. Split-rib cranioplasty using a patient-specific three-dimensional printing model. Arch Plast Surg. 2016. 43: 379-81
11. Lloret I, Server A, Taksdal I. Calvarial lesions: A radiological approach to diagnosis. Acta Radiol. 2009. 50: 531-42
12. Meekeren JJ, editors. Observationes medico-Chirugicae. Amsterdam: Ex Officina Henrici and Vidnae Theodori Boom; 1682. p.
13. Merola J, Muthu T, Hussain Z, Lamont D. Case report: Destructive neuroendocrine cranial tumour and the role of pre-fashioned polyetheretherketone (PEEK) cranioplasty. Open Cancer J. 2012. 5: 7-10
14. Mohan D, Munteanu V, Moisa H, Ciurea AV. A medical insight on the of biomaterials for cranioplasty surgery. Key Eng Mater. 2015. 638: 205-9
15. Morales-Gómez JA, Garcia-Estrada E, Leos-Bortoni JE, Delgado-Brito M, Flores-Huerta LE, Adriana A. Cranioplasty with a low-cost customized polymethylmethacrylate implant using a desktop 3D printer. J Neurosurg. 2018. 130: 1721-7
16. Moreira-Gonzalez A, Jackson IT, Miyawaki T, Barakat K, DiNick V. Clinical outcome in cranioplasty: Critical review in long-term follow-up. J Craniofac Surg. 2003. 14: 144-53
17. Park EK, Lim JY, Yun IS, Kim JS, Woo SH, Kim DS. Cranioplasty Enhanced by three-dimensional printing: Custom-made three-dimensional-printed titanium implants for skull defects. J Craniofac Surg. 2016. 27: 943-9
18. Scolozzi P, Martinez A, Jaques B. Complex orbito-fronto-temporal reconstruction using computer-designed PEEK implant. J Craniofac Surg. 2007. 18: 224-8
19. Wheeler DL, Eschbach EJ, Hoellrich RG, Montfort MJ, Chamberland DL. Assessment of resorbable bioactive material for grafting of critical-size cancellous defects. J Orthop Res. 2000. 18: 140-8
20. Yaremchuk MJ. Facial skeletal reconstruction using porous polyethylene implants. Plast Reconstr Surg. 2003. 111: 1818-27
21. Zanotti B, Zingaretti N, Verlicchi A, Robiony M, Alfieri A, Parodi PC. Cranioplasty: Review of materials. J Craniofac Surg. 2016. 27: 2061-72
22. Zegers T, Laak-Poort M, Koper D, Lethaus B, Kessler P. The therapeutic effect of patient-specific implants in cranioplasty. J Craniomaxillofac Surg. 2017. 45: 82-6
23. Zhang J, Tian W, Chen J, Yu J, Zhang J, Chen J. The application of polyetheretherketone (PEEK) implants in cranioplasty. Brain Res Bull. 2019. 153: 143-9