- Department of Neurosurgery, Loma Linda University Medical School, Loma Linda, California, United States
- Department of Neurosurgery, Loma Linda University Medical Center, Loma Linda, California, United States
- Department of Pathology, Loma Linda University Medical Center, Loma Linda, California, United States
Correspondence Address:
Justin Dye, Department of Neurosurgery, Loma Linda University Medical Center, Loma Linda, California, United States.
DOI:10.25259/SNI_259_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Brandon Michael Edelbach1, Vadim Gospodarev2, Ravi Raghavan3, Justin Dye2. Primary intracranial sarcoma, DICER-1 mutant, with hemorrhagic presentation: A case report. 26-Jul-2024;15:253
How to cite this URL: Brandon Michael Edelbach1, Vadim Gospodarev2, Ravi Raghavan3, Justin Dye2. Primary intracranial sarcoma, DICER-1 mutant, with hemorrhagic presentation: A case report. 26-Jul-2024;15:253. Available from: https://surgicalneurologyint.com/surgicalint-articles/13017/
Abstract
Background: Primary intracranial sarcomas (PIS) are rare tumors with mesenchymal origins. These tumors have a heterogeneous clinical presentation and are associated with a poor prognosis.
Case Description: This report highlights the complexities associated with PIS by focusing on a 26-year-old male with recurrent tumor growth facing unique challenges regarding diagnosis and treatment options . A high-grade spindle-celled neoplasm with sarcomatous features characterized the patient’s tumor. There were additional morphologic changes, including multinucleated giant cells and rare foci with eosinophilic spheroids. Genomic analysis revealed a DICER1-associated PIS. Treatment involved endovascular embolization, multiple surgical interventions, intrathecal etoposide injections, and oral pazopanib with adjuvant radiation therapy.
Conclusion: This case additionally highlights an unusual association between PIS and anomalous hypervascularity, refractory hemorrhage, and subdural effusions, a presentation that is increasingly being reported in this type of tumor.
Keywords: DICER-1, Hematoma, Primary intracranial sarcoma, Tumor, Vascular fistula, DICER-1 mutation, Vascular malformation
INTRODUCTION
Primary intracranial sarcomas (PIS) are rare tumors that originate from multipotent primitive mesenchymal cells within leptomeninges or dura.[
The prognosis of PIS is poor, with an overall 5-year survival rate of 33.4% with a mean survival time estimated at 15.5 months.[
Diagnosis of PIS is typically based on clinical, pathological, and imaging features. Common imaging findings include contrast enhancement, diffusion restriction, hemorrhage, meningeal extension, and necrosis.[
Treatment often includes a combination of surgery, radiotherapy, and chemotherapy.[
CASE PRESENTATION
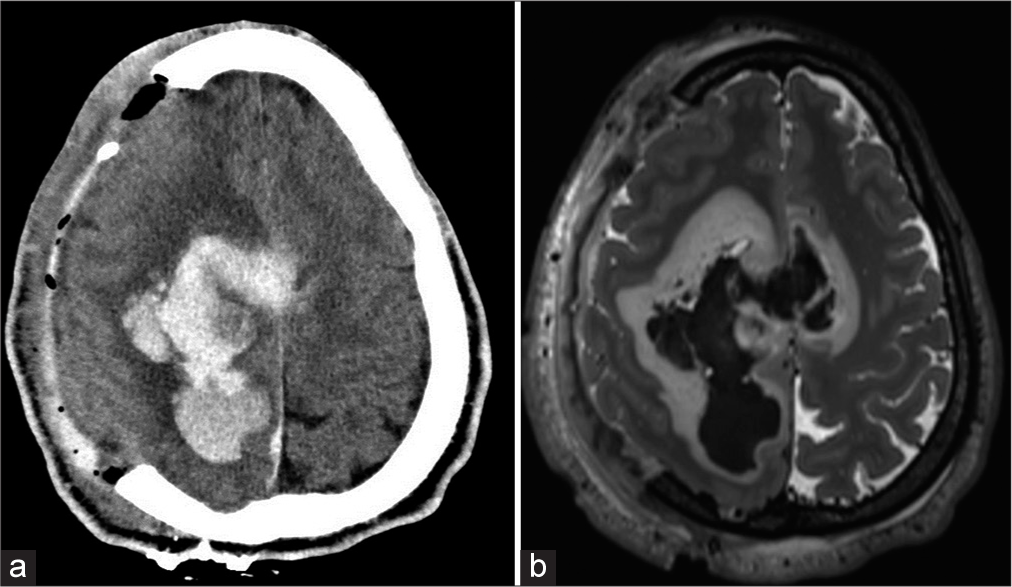
The patient was a 26-year-old male with a history of anxiety, alcohol, and cannabis use disorder who presented at an outside institution for severe headaches. Computed tomography (CT) and magnetic resonance imaging (MRI) revealed right frontoparietal intracranial hemorrhage with mass effect requiring emergent decompressive craniectomy [
While no definite evidence of AVM was encountered on the first DSA, there was still concern for an underlying vascular malformation that the large hemorrhage may have compressed. An underlying hemorrhagic tumor was also considered in the differential; however, the initial MRI showed only lobulated areas of enhancement along the margin of the hemorrhage. To evaluate the possibility of metastatic disease, the patient received CT imaging of the chest, abdomen, and pelvic regions, which were negative. As the patient was neurologically stable and had already undergone decompression, no further surgical intervention was performed, and the patient was eventually transferred to rehab.
One month later, the patient was re-presented with acute onset nausea, vomiting, increased lethargy, and aphasia. Repeat imaging revealed an increased size of the right frontoparietal hematoma [
Given the repeat hemorrhage, persistent neurologic deficits, and mass effect, the patient was brought to the operating room for evacuation of the hemorrhage and resection of any underlying cause. Through a right frontal craniotomy, the hematoma was evacuated, and an abnormal mass was encountered deep within the right frontal lobe. The mass was biopsied, and intraoperative pathology was concerning for the malignant tumor. The tumor was debulked down to the ventricular surface, and near total resection was achieved.
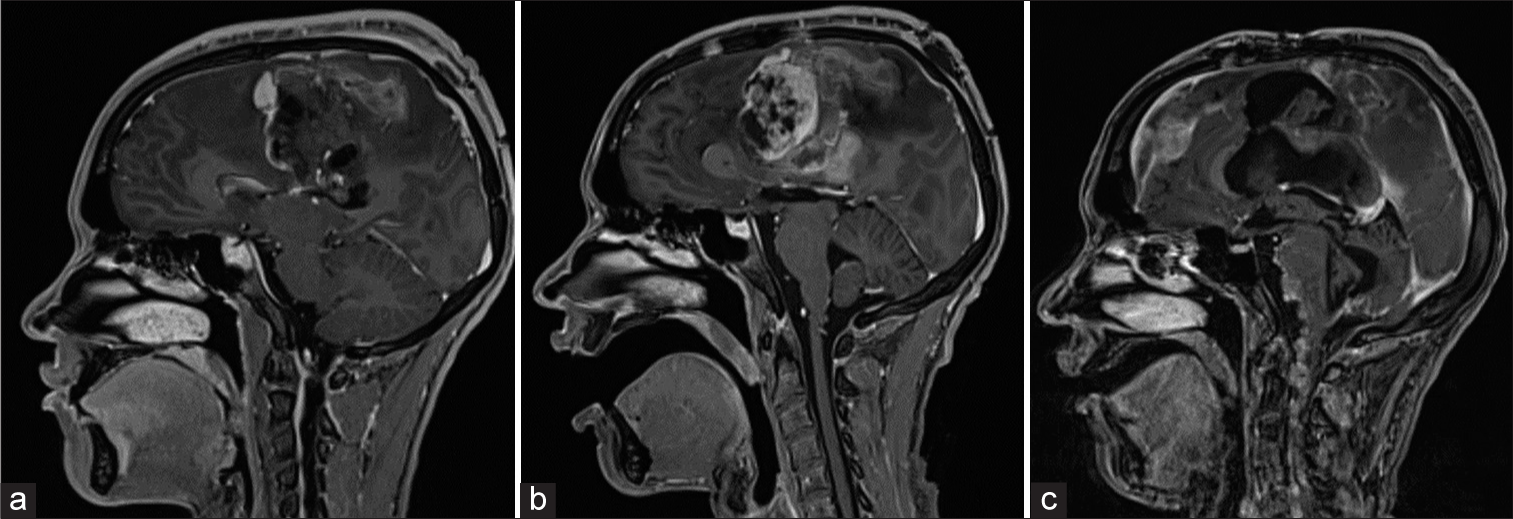
Immediate postoperative imaging showed a residual tumor centered at the left paramedian frontal lobe [
Figure 4:
(a) T1-weighted magnetic resonance imaging (MRI) demonstrating post-surgical changes with residual enhancing tumor along the margin of the surgical bed. (b) T1-weighted MRI showing residual tumor growth 2 weeks later. Heterogenous, multilobulated tumor now measuring 6.8 × 6.2 × 6.3 cm. (c) T1-weighted magnetic resonance imaging demonstrating diffuse leptomeningeal enhancement.
Pathology
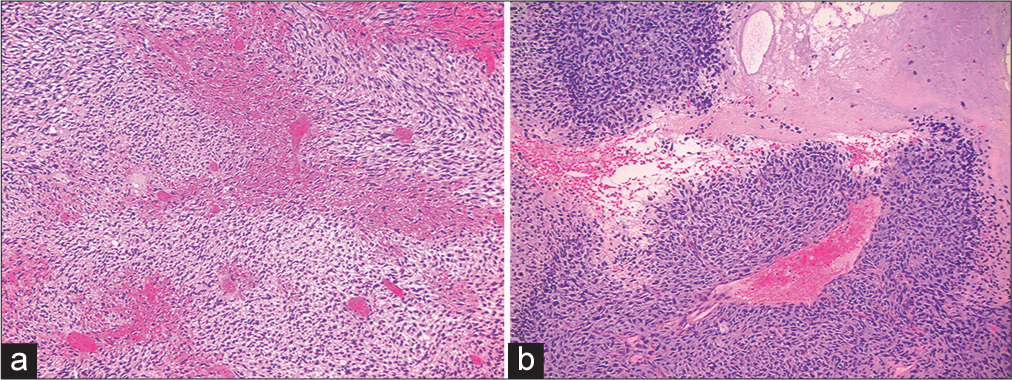
Initial histological examination of the tumor revealed a high-grade spindle-celled neoplasm with sarcomatous features (World Health Organization [WHO] grade IV). There were additional areas with pleomorphic changes, including multinucleated giant cells, eosinophilic spheroids, and regions with a fibrillary background [
Figure 5:
(a-c) Histological demonstration of resected tumor demonstrating a highly cellular (>80%), spindle celled, sarcomatous neoplasm with a fibrillary background, regional pseudopalisading necrosis, and pleomorphic changes including multinucleated, bizarre giant cells, and eosinophilic spheroids.
On immunohistochemistry (IHC) staining, the tumor was positive for vimentin, with patchy reactivity for reticulin. However, since there was no clear indication of its lineage or subtype, samples were sent for genomic analysis, and deoxyribonucleic acid methylation profiling was performed. The methylation profile on the Heidelberg classifier (version 11b6 and 12b6) and National Cancer Institute/Bethesda classifier indicated a high-grade PIS DICER1-mutant. Further, tumor profiling was pursued and a notable biomarker included a pathogenic variant on platelet-derived growth factor receptor alpha.
Adjuvant therapy
After the second surgery for tumor resection, the patient received five fractions of radiation utilizing intensity-modulated radiation therapy. Chemotherapy options were also discussed with the patient and his family. However, the patient was not willing to receive blood products based on religious beliefs, which limited aggressive chemotherapy approaches. Thus, the traditional approach of treating intracranial sarcomas with ICE was avoided due to the risk of myelosuppression. Instead, the patient began treatment with etoposide biweekly for 6 weeks (0.5 mg, intrathecal). The patient tolerated etoposide well, and due to the molecular profile of the patient’s tumor, oral pazopanib was added to the patient’s regimen.
DISCUSSION
The purpose of this report is to highlight a relatively new entity described in the 2021 WHO central nervous system (CNS) five classifications of CNS tumors that pose challenges in clinical and morphologic diagnosis and require additional molecular investigations for confirmation. This entity also tends to present with catastrophic hemorrhage at times and is often seen in younger individuals. Besides the obvious spindle-celled pleomorphic architecture of a sarcoma, one additional hallmark is the finding of eosinophilic globules, although their presence can be patchy and may be easily overlooked without extensive sampling and evaluation in multiple sections. The index case itself did not reveal these eosinophilic globules in the initial resection, and only a detailed analysis of a subsequent resection specimen revealed the abnormality in a small focus.
DICER1 mutation syndrome is an autosomal dominant predisposition to several types of cancer secondary to a mutation in an RNase III endonuclease.[
Koelsche et al. proposed a subtype of PIS termed spindle cell sarcoma with rhabdomyosarcoma-like features (SCS-RMSDICER1), which had a significant association with DICER1 mutation in 22 cases.[
Kamihara et al. reported DICER1-associated CNS sarcomas, which had a significant association with DICER1 mutation in six cases. These tumors were spindle cells with brisk mitotic activity in a fascicular background. There was evidence of eosinophilic globules and regions of cartilaginous formations. These cells were poorly differentiated but showed focal positivity for myogenic markers. Finally, there was evidence of palisading necrosis in one of the patients.[
Finally, Yao et al. described a third set of DICER1-associated PIS that bore similarity to the histological description of the other subclassifications of DICER1-associated PIS that showed dense, spindled cells with eosinophilic globules. However, Yao et al. noted that PIS has a unique positive IHC for neurogenic markers.[
The significant hypervascularity and large ICH associated with the tumor presented in this case report created significant difficulties in diagnosis. The association of PIS with subdural effusions or hematomas is not unusual, although there are only limited descriptions in the literature. As pathologists and clinicians recognize more awareness of this entity and its association with the DICER-1 alteration, more anecdotal (but unpublished) descriptions are reported. Of the 16 patients reported by Al-Gahtany et al., three were reported to have tumors with associated hypervascularity on angiogram, with an additional patient reported to have intratumoral and intraventricular hemorrhage mimicking the appearance of an AVM on imaging.[
CONCLUSION
DICER1-associated PIS is a rare, characteristically heterogeneous subset of sarcomas. These intracranial sarcomas have been described with myogenic, chondroid, and neurogenic differentiation. Despite advancements in understanding the molecular landscape of these tumors, the overall prognosis for DICER1-associated PIS remains poor, emphasizing the urgency for continued research. Furthermore, there appears to be a noteworthy association between PIS and anomalous hypervascularity, resulting in these tumors presenting as hemorrhagic lesions, adding a level of complexity to the diagnosis and management of these difficult cases.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Alexandrescu S, Meredith DM, Lidov HG, Alaggio R, Novello M, Ligon KL. Loss of histone H3 trimethylation on lysine 27 and nuclear expression of transducin-like enhancer 1 in primary intracranial sarcoma, DICER1-mutant. Histopathology. 2021. 78: 265-75
2. Al-Gahtany M, Shroff M, Bouffet E, Dirks P, Drake J, Humphreys R. Primary central nervous system sarcomas in children: Clinical, radiological, and pathological features. Childs Nerv Syst. 2003. 19: 808-17
3. Anglesio MS, Wang Y, Yang W, Senz J, Wan A, HeraviMoussavi A. Cancer-associated somatic DICER1 hotspot mutations cause defective miRNA processing and reverse-strand expression bias to predominantly mature 3p strands through loss of 5p strand cleavage. J Pathol. 2013. 229: 400-9
4. Bailey OT, Ingraham FD. Intracranial fibrosarcomas of the Dura mater in childhood: Pathological characteristics and surgical management. J Neurosurg. 1945. 2: 1-15
5. Cai YX, Liu JS, Xu J, He YZ, Zhang HN, Tian SF. Primary intracranial sarcomas: A clinicopathological investigation. Front Oncol. 2023. 13: 1195467
6. Cinalli G, Zerah M, Carteret M, Doz F, Vinikoff L, LellouchTubiana A. Subdural sarcoma associated with chronic subdural hematoma. Report of two cases and review of the literature. J Neurosurg. 1997. 86: 553-7
7. Das A, Roy P, Modi SK, Achari RB, Sen S, Singh A. Germline DICER1-mutant intracranial sarcoma with dual chondroid and spindle cell morphology and pulmonary metastases treated with multimodal therapy. Pediatr Blood Cancer. 2019. 66: e27744
8. De Kock L, Sabbaghian N, Plourde F, Srivastava A, Weber E, Bouron-Dal Soglio D. Pituitary blastoma: A pathognomonic feature of germ-line DICER1 mutations. Acta Neuropathol. 2014. 128: 111-22
9. Diaz Coronado RY, Mynarek M, Koelsche C, Mora Alferez P, Casavilca Zambrano S, Wachtel Aptowitzer A. Primary central nervous system sarcoma with DICER1 mutation-treatment results of a novel molecular entity in pediatric Peruvian patients. Cancer. 2022. 128: 697-707
10. Haider AS, Palmisciano P, Sagoo NS, Bin Alamer O, El Ahmadieh TY, Pan E. Primary central nervous system sarcomas in adults: A systematic review. Clin Neurol Neurosurg. 2022. 214: 107127
11. Kamihara J, Paulson V, Breen MA, Laetsch TW, Rakheja D, Shulman DS. DICER1-associated central nervous system sarcoma in children: Comprehensive clinicopathologic and genetic analysis of a newly described rare tumor. Modern Pathol. 2020. 33: 1910-21
12. Koelsche C, Mynarek M, Schrimpf D, Bertero L, Serrano J, Sahm F. Primary intracranial spindle cell sarcoma with rhabdomyosarcoma-like features share a highly distinct methylation profile and DICER1 mutations. Acta Neuropathol. 2018. 136: 327-37
13. Lafay-Cousin L, Lindzon G, Taylor MD, Hader W, Hawkins C, Nordal R. Successful treatment of primary intracranial sarcoma with the ICE chemotherapy regimen and focal radiation in children. J Neurosurg Pediatr. 2016. 17: 298-302
14. Lazarte-Rantes C, Pillaca-Cruzado O, Baca-Hinojosa N, Mamani W, Lee-Diaz J, Ugas-Charcape CF. MRI findings of primary intracranial sarcomas in children. Pediatr Radiol. 2023. 53: 1698-703
15. Maher OM, Khatua S, Mukherjee D, Olar A, Lazar A, Luthra R. Primary intracranial soft tissue sarcomas in children, adolescents, and young adults: Single institution experience and review of the literature. J Neurooncol. 2016. 127: 155-63
16. Nejat F, Keshavarzi S, Monajemzadeh M, Mehdizadeh M, Kalaghchi B. Chronic subdural hematoma associated with subdural rhabdomyosarcoma: Case report. Neurosurgery. 2007. 60: E774-5 discussion E775
17. Oliveira AM, Scheithauer BW, Salomao DR, Parisi JE, Burger PC, Nascimento AG. Primary sarcomas of the brain and spinal cord: A study of 18 cases. Am J Surg Pathol. 2002. 26: 1056-63
18. Sakaguchi M, Nakano Y, Honda-Kitahara M, Kinoshita M, Tanaka S, Oishi M. Two cases of primary supratentorial intracranial rhabdomyosarcoma with DICER1 mutation which may belong to a “spindle cell sarcoma with rhabdomyosarcoma-like feature, DICER1 mutant. Brain Tumor Pathol. 2019. 36: 174-82
19. Schultz KA, Williams GM, Kamihara J, Stewart DR, Harris AK, Bauer AJ. DICER1 and associated conditions: Identification of at-risk individuals and recommended surveillance strategies. Clin Cancer Res. 2018. 24: 2251-61
20. Slade I, Bacchelli C, Davies H, Murray A, Abbaszadeh F, Hanks S. DICER1 syndrome: Clarifying the diagnosis, clinical features and management implications of a pleiotropic tumour predisposition syndrome. J Med Genet. 2011. 48: 273-8
21. Vasoya P, Raj V, Khan K, Thakar S, Aryan S. Primary intracranial sarcoma masquerading as a chronic subdural haematoma: Illustrative case and review of an unusual phenomenon. Childs Nerv Syst. 2023. 39: 1957-62
22. Yao K, Duan Z, Feng J, Yan C, Qi X. DICER1-associated central nervous system sarcoma with neural lineage differentiation: A case report. Diagn Pathol. 2022. 17: 72
23. Zhang G, Xiao B, Huang H, Zhang Y, Zhang X, Zhang J. Intracranial synovial sarcoma: A clinical, radiological and pathological study of 16 cases. Eur J Surg Oncol. 2019. 45: 2379-85