- Department of Neurosurgery, Memfys Hospital for Neurosurgery, Onitsha Expressway, Enugu, Nigeria,
- Department of Neurosurgery, Shinshu University School of Medicine, Asahi, Matsumoto, Nagano, Japan.
- Department of Laboratory Medicine, Shinshu University School of Medicine, Asahi, Matsumoto, Nagano, Japan.
Correspondence Address:
Kohei Kanaya
Department of Neurosurgery, Shinshu University School of Medicine, Asahi, Matsumoto, Nagano, Japan.
DOI:10.25259/SNI_170_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Tobechi Nwankwo Mbadugha, Kohei Kanaya, Tetsuyoshi Horiuchi, Mai Iwaya, Samuel Chukwunonyerem Ohaegbulam, Kazuhiro Hongo. Primary myxoid temporal bone tumor: A rare neurosurgical manifestation of Carney complex?. 27-Jun-2020;11:166
How to cite this URL: Tobechi Nwankwo Mbadugha, Kohei Kanaya, Tetsuyoshi Horiuchi, Mai Iwaya, Samuel Chukwunonyerem Ohaegbulam, Kazuhiro Hongo. Primary myxoid temporal bone tumor: A rare neurosurgical manifestation of Carney complex?. 27-Jun-2020;11:166. Available from: https://surgicalneurologyint.com/surgicalint-articles/10102/
Abstract
Background: Carney complex (CNC) is a rare autosomal dominant syndrome, manifesting mainly with cardiac, cutaneous, and mucosal myxomas. Osteochondromyxoma is known as an extremely rare bone lesion of CNC which usually appears early in life; however, there were no reports of primary bone myxoma of the skull in the patients with CNC. We present the first case of primary myxoid skull tumor in the patient with CNC.
Case Description: We report the left temporal bone tumor with significant intracranial mass effect in a 58-year- old woman already diagnosed with CNC. Complete resection of the tumor with skull bone reconstruction was carried out. Pathological diagnosis was labeled the lesion as an atypical myxoid spindle cell neoplasm. The features were different from atrial myxoma and osteochondromyxoma which has been described in CNC. There have been no signs of recurrence in 9 years follow-up.
Conclusion: To the best of our knowledge, there have been no reports of the primary myxoid tumors in the skull in the patients with CNC. This paper highlighted a possible important association between CNC and primary intracranial myxoid tumors.
Keywords: Carney complex, Myxoid tumor, Skull
INTRODUCTION
Carney complex (CNC) is a rare autosomal dominant syndrome characterized by pigmented, myxomatous lesions of the skin, mucosae, cardiac, and other tissues as well as multiple endocrine and nonendocrine neoplasms.[
Primary myxoid tumors rarely occur intracranially. To the best of our knowledge, there have been no previous reports in the literature of primary myxoid tumors of the head-and-neck region identified in CNC patients.
We present a 58-year-old woman with a primary myxoid tumor in the left temporal bone in the setting of CNC diagnosis. This case illustrates the challenges in making a histological diagnosis in this group of tumors and the features that make them difficult to treat radically, as previously reported.[
CASE REPORT
History and examination
A 58-year-old woman presented with a 4-month history of anomic aphasia, memory disturbance, and a recent onset of dizziness. At age 32 years, she was managed for stroke resulting from atrial myxoma, which was removed surgically. The patient’s daughter and granddaughter were both diagnosed with CNC, in addition to the daughter having Cushing’s syndrome.
On examination, she was fully conscious and cooperative. She had fluent aphasia as well as naming disorder and agraphia. Pupils were 2.5 mm in size bilaterally and promptly reactive, and there were no obvious cranial neuropathies. Motor and sensory examinations were unremarkable, and cerebellar signs were negative. The other system examinations were essentially normal.
Radiological findings
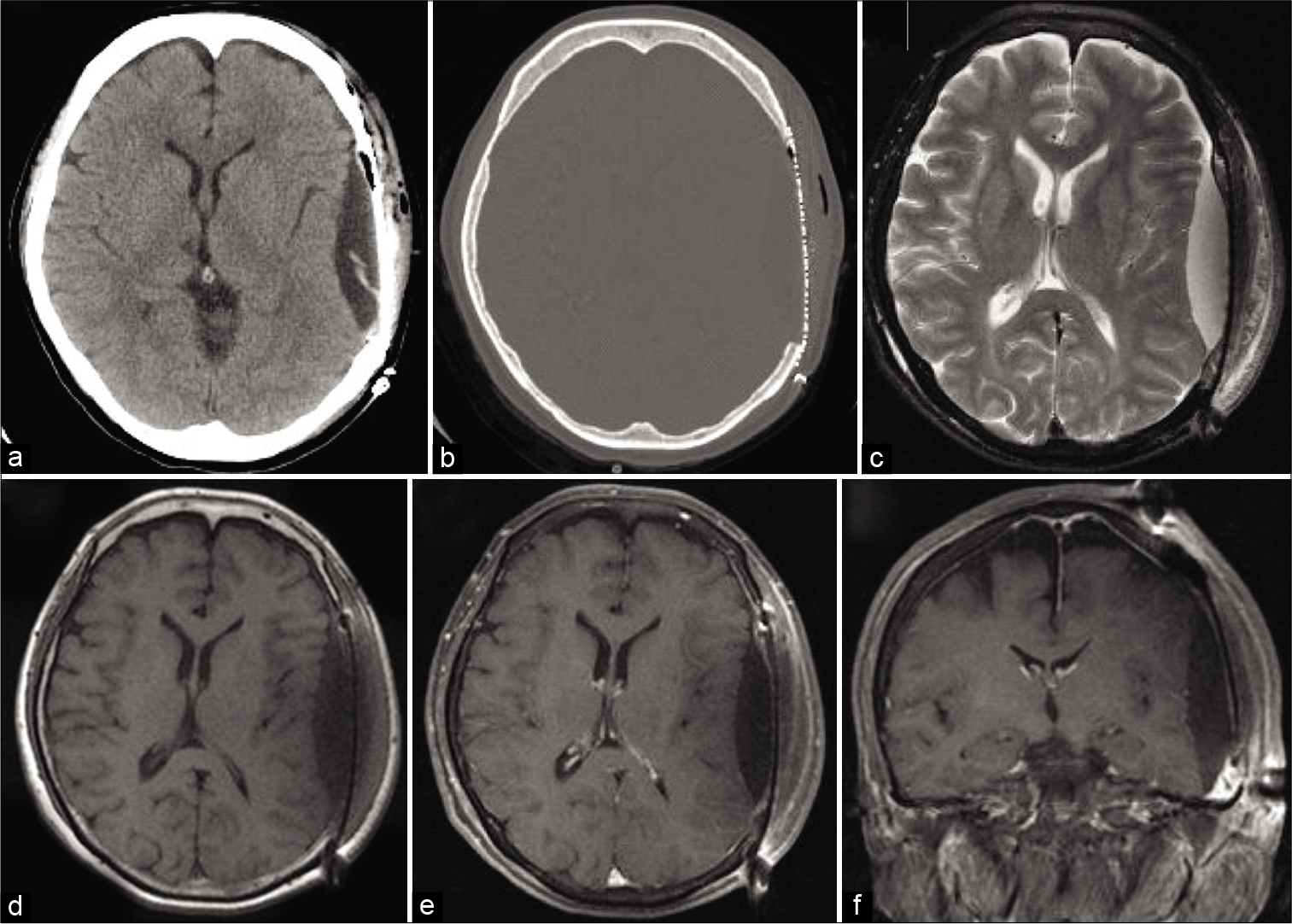
Computed tomography (CT) scan showed a well-circumscribed, hypodense, extra-axial mass in the left temporal region with significant bony erosion and partially calcified rims. Brain magnetic resonance imaging (MRI) revealed a heterogeneously contrast-enhanced mass with significant displacement of the adjacent temporoparietal lobes [
Figure 1:
Axial brain CT scans showing the hypodense mass with partially calcified rim and bone erosion of the adjacent left temporal bone. (a,b) Axial T2-weighted MR image showing hyperintense lesion at the left temporal bone. (c) Axial T1-weighted MR image showing hypointense circumferential mass and hyperintense fluid component in the mass. (d) Axial and coronal enhanced T1-weighted MR images showing heterogeneous contrast enhancement of the tumor. (e,f)
Treatment and postoperative course
The patient underwent tumor excision through a left frontotemporal approach. There was tumor invasion of the cranium and partial destruction of the outer table of the skull; however, the temporalis muscle and periosteum were intact. The tumor was consisted of a yellowish soft lesion with a jelly-like component. The inner table of the skull was destroyed, and the tumor was adherent to the dura. The tumor was meticulously dissected from the dura and gross total resection of the tumor was achieved [
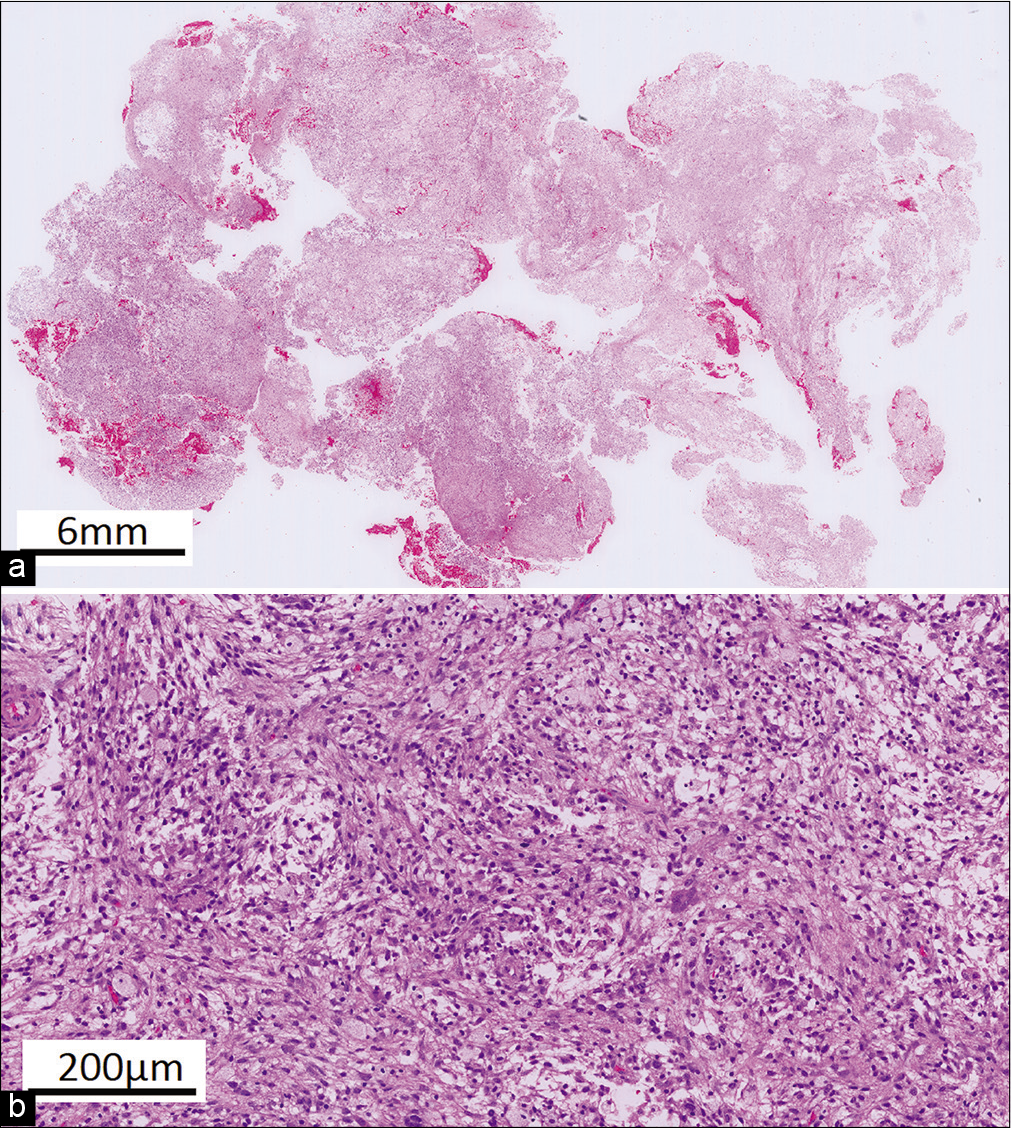
Histopathological diagnosis
Grossly, the pathological specimen consisted of a yellowish, soft, and gelatinous tissue. Histologically, the resected lesion showed a proliferation of bland spindle cells which had ovoid to elongated nuclei with myxoid stroma. Occasionally, spindle cells with atypical hyperchromic nuclei were seen. There were prominent macrophages and no cartilage, osteoid, or bone were identified [
DISCUSSION
Primary myxoma, a benign lesion of mesenchymal origin, is a rare intracranial lesion. Metastatic/embolic atrial myxoma, although uncommon, is a more frequent occurrence than primary myxoid intracranial tumors.[
Of the cases of primary myxomas reported in the head-and- neck region, the most common locations were the maxilla and mandible.[
Unlike the true myxomas, the other myxoid bone tumors are more malignant lesions with myxomatous degeneration. They lack differentiated mesenchymal elements with hypercellularity, nuclear pleomorphism, and mitotic figures. The differentials include myxoid chondrosarcoma, myxoliposarcoma, myxorhabdomyosarcoma, chordoma, myxoid malignant fibrous histiocytoma, and metastatic tumors of the skull.[
Immunohistochemistry may be helpful in distinguishing these myxoid lesions, although the findings were inconsistent with the index case. Findings supporting a myxoma is positive staining for vimentin and negative for S-100, desmin, neuron-specific enolase, keratin, and glial fibrillary acid protein. It also shows positivity for α-smooth muscle actin (SMA), calretinin and CD34 of cardiac myxomas, and less for epithelial membrane antigen (EMA) [
On the whole, the recognition of a possible association between CNC and a primary intracranial bone myxoma has implications in patient management, bearing in mind the known risks in the perioperative management of patients with CNC. They are at risk for ACTH-independent Cushing’s syndrome and cardiac myxoma with its subsequent risk of hemodynamic or embolic complications, including stroke and heart failure.[
More so, making an accurate diagnosis will help in genetic screening and counseling of both the patient and relatives as they are predisposed to certain tumors such as growth hormone-secreting adenomas and nerve sheath tumors, including schwannomas which are more common neurosurgical manifestations of CNC.[
The treatment of primary intracranial myxoid tumors is surgical removal as the tumor is generally insensitive to radiation.[
Fortunately, the temporal convexity was involved in the index case, and the dura was not bridged even though the tumor was adherent. Thus, the tumor was meticulously detached from the dura and completely resected with the removal of the involved convexity bone. Cranioplasty was subsequently performed to repair the deformity. This would have been more challenging in the skull base with an increased risk of serious complications such as cerebrospinal fluid leak and cranial nerve injuries.
Recurrence of primary myxoid bone tumors is common, with 25% of tumors recurring if they were not radically resected.[
CONCLUSION
We reported a case of primary myxoid skull tumor in the patient with CNC treated with radical resection without recurrence in 9 years follow-up. As previously reported[
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Andrews T, Kountakis SE, Maillard AA. Myxomas of the head and neck. Am J Otolaryngol. 2000. 21: 184-9
2. Bernet F, Stulz PM, Carrel TP. Long-term remission after resection, chemotherapy, and irradiation of a metastatic myxoma. Ann Thorac Surg. 1998. 66: 1791-2
3. Burke A, Tavora F. The 2015 WHO classification of tumors of the heart and pericardium. J Thorac Oncol. 2015. 11: 441-52
4. Charabi S, Engel P, Bonding P. Myxoid tumours in the temporal bone. J Laryngol Otol. 1989. 103: 1206-9
5. Correa R, Salpea P, Stratakis C. Carney Complex : An update. Eur J Endocrinol. 2015. 173: M85-97
6. Hsieh DL, Tseng HM, Young YH. Audiovestibular evolution in a patient undergoing surgical resection of a temporal bone myxoma. Eur Arch Otorhinolaryngol. 2006. 263: 614-7
7. Klein MV, Schwaighofer BW, Sobel DF, Hesselink JR. Primary myxoma of the posterior fossa. Neuroradiology. 1990. 32: 250-1
8. Nagatani M, Mori S, Takimoto N, Arita N, Ushio Y, Hayakawa T. Primary myxoma in the pituitary fossa: Case report. Neurosurgery. 1987. 20: 329-31
9. Oruckaptan HH, Sarac S, Gedikoglu G. Primary intracranial myxoma of the lateral skull base: A rare entity in clinical practice. Turk Neurosurg. 2010. 20: 86-9
10. Osterdock RJ, Greene S, Mascott CR, Amedee R, Crawford BE. Primary myxoma of the temporal bone primary myxoma of the temporal bone in a 17-year-old boy : Case report. Neurosurgery. 2001. 48: 945-8
11. Rupp GM, Heyman RA, Martinez AJ, Sekhar LN, Jungreis CA. The pathology of metastatic cardiac myxoma. Am J Clin Pathol. 1989. 91: 221-7
12. Sekhar LN, Pomeranz S, Janecka IP, Hirsch B, Ramasastry S. Temporal bone neoplasms: A report on 20 surgically treated cases. J Neurosurg. 1992. 76: 578-87
13. Singhal P, Luk A, Rao V, Butany J. Molecular basis of cardiac myxomas. Int J Mol Sci. 2014. 151: 1315-37
14. Vezzosi D, Vignaux O, Dupin N, Bertherat J. Carney complex: Clinical and genetic 2010 update. Ann Endocrinol (Paris). 2010. 71: 486-93
15. Watson JC, Stratakis CA, Bryant-Greenwood PK, Koch CA, Kirschner LS, Nguyen T. Neurosurgical implications of carney complex. J Neurosurg. 2000. 92: 413-8
16. Whitman RA, Stewart S, Stoopack JG, Jerrold TL. Myxoma of the mandible: Report of case. J Oral Surg. 1971. 29: 63-70