- Department of Neurosurgery, Kansas University, Kansas City, Missouri,
- Department of Neurosurgery, University of Louisville, Louisville, Kentucky, United States.
Correspondence Address:
Zaid Aljuboori
Department of Neurosurgery, University of Louisville, Louisville, Kentucky, United States.
DOI:10.25259/SNI_482_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Michael Kinsman1, Zaid Aljuboori2, Tyler Ball2, Haring Nauta2, Maxwell Boakye2. Rapid high-fidelity contour shaping of titanium mesh implants for cranioplasty defects using patient-specific molds created with low-cost 3D printing: A case series. 12-Sep-2020;11:288
How to cite this URL: Michael Kinsman1, Zaid Aljuboori2, Tyler Ball2, Haring Nauta2, Maxwell Boakye2. Rapid high-fidelity contour shaping of titanium mesh implants for cranioplasty defects using patient-specific molds created with low-cost 3D printing: A case series. 12-Sep-2020;11:288. Available from: https://surgicalneurologyint.com/surgicalint-articles/10252/
Abstract
Background: Cranioplasty is a neurosurgical procedure to repair skull defects. Sometimes, the patients’ bone flap cannot be used for various reasons. Alternatives include a custom polyether ether ketone (PEEK) implant or titanium mesh; both incur an additional cost. We present a technique that uses a 3D printer to create a patient- specific 3D model used to mold a titanium mesh preoperatively.
Case Description: We included three patients whose bone flap could not be used. We collected the patients’ demographics, cost, and time data for implants and the 3D printer. The patients’ computed tomography DICOM images were used for 3D reconstruction of the cranial defect. A 3D printer (Flashforge, CA) was used to print a custom mold of the defect, which was used to shape the titanium mesh. All patients had excellent cosmetic results with no complications. The time required to print a 3D model was ~ 6 h and 45 min for preoperative shaping of the titanium implant. The intraoperative molding (IOM) of a titanium mesh needed an average of 60 min additional operative room time which incurred $4000. The average cost for PEEK and flat titanium mesh is $12,600 and $6750. Our method resulted in $4000 and $5500 cost reduction in comparison to flat mesh with IOM and PEEK implant.
Conclusion: 3D printing technology can create a custom model to shape a titanium mesh preoperatively for cranioplasty. It can result in excellent cosmetic results and significant cost reduction in comparison to other cranioplasty options.
Keywords: 3D printing, Cranioplasty, Custom, Polyether ether ketone, Surgical economics, Titanium
INTRODUCTION
Decompressive craniectomy is a potentially life-saving operation for patients with cerebral edema resulting from traumatic brain injury, stroke, or intracerebral hemorrhage.[
CASE DESCRIPTION
We included three patients whose bone flap could not be used for various reasons. We collected the patients’ demographics as well as the time and cost data of the cranioplasty implants, 3D printer, IOM, and operating room (OR) usage. We summarized data using means, ranges, and percentages.
The technique for preparing the 3D mold
The patients’ pre- and post-hemicraniectomy CT scans DICOM images were imported into Osirix (Pixmeo, Switzerland) and converted into a 3D model, which was then exported to MeshLab for editing and 3D reconstruction of the cranial defect. A MakerBot (MakerBot, NY) software was then used to convert the files into a form compatible with the 3D printer. A Flashforge Creator Pro 3D printer (Flashforge, CA) was used to print a custom mold of the cranial defect for each patient. We used the custom mold to shape and size a 1 mm thickness Stryker Leibinger flat titanium mesh (200x200 mm) (Stryker, MI), which was then sent for sterilization and storage before the day of surgery [
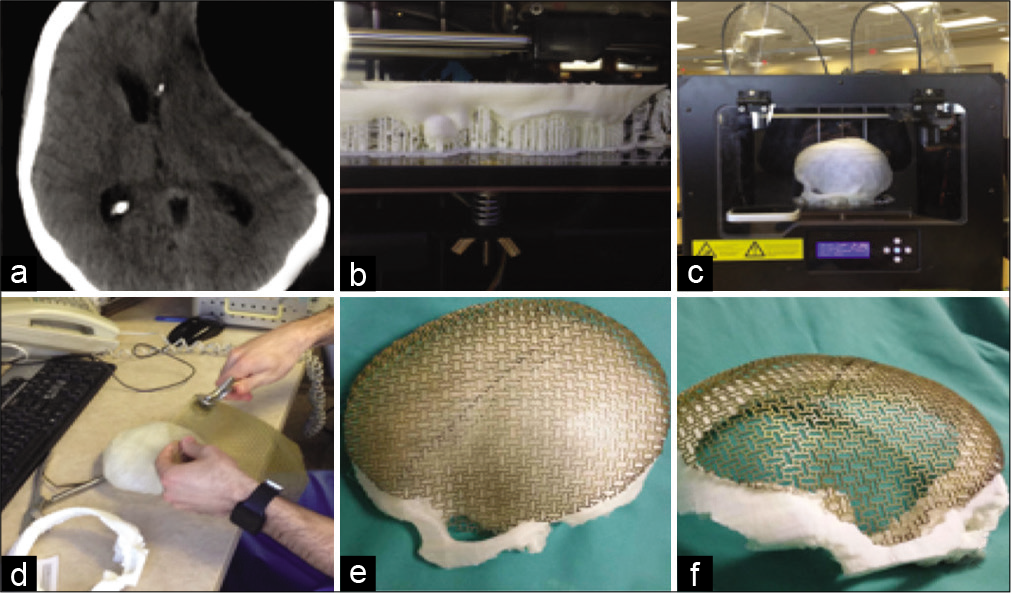
Figure 1:
(a) Postdecompressive craniectomy CT image showing the cranial defect. (b) Early and (c) late stages of the 3D printing of the 3D model. (d) A picture shows the shaping of a 1 mm thick titanium mesh using a shaping tool and the 3D-printed mold (e) and (f) images of the shaped titanium mesh conforming to the 3D model and the bony defect.
CASE ILLUSTRATIONS
Case 1
A 49-year-old male with a history of a gunshot wound to the head that resulted in left-sided acute subdural hematoma requiring a decompressive hemicraniectomy. Because of concerns over the infection and extensive damage to the patient’s skull from the gunshot, the patient’s bone flap was not able to be used for cranioplasty. Therefore, we used the 3D printer to print a custom model to mold the titanium mesh preoperatively.
Case 2
A 23-year-old male with a history of severe traumatic brain injury due to a motor vehicle accident that required a hemicraniectomy. The initial cranioplasty was done using the patient’s bone flap, but the patient developed a wound infection with extension into the bone flap that required revision with the disposal of the bone flap. After the infection was treated, we used the 3D-printed custom mold to shape the titanium mesh preoperatively.
Case 3
A 17-year-old male with a history of a gunshot wound to the head that required a bifrontal decompressive craniectomy. His frontal bone was shattered by the injury and was not suitable for cranioplasty. Therefore, we used a 3D-printed mold to shape the titanium mesh preoperatively.
RESULTS
All three procedures were uneventful with no complications, and the patients had excellent cosmetic results [
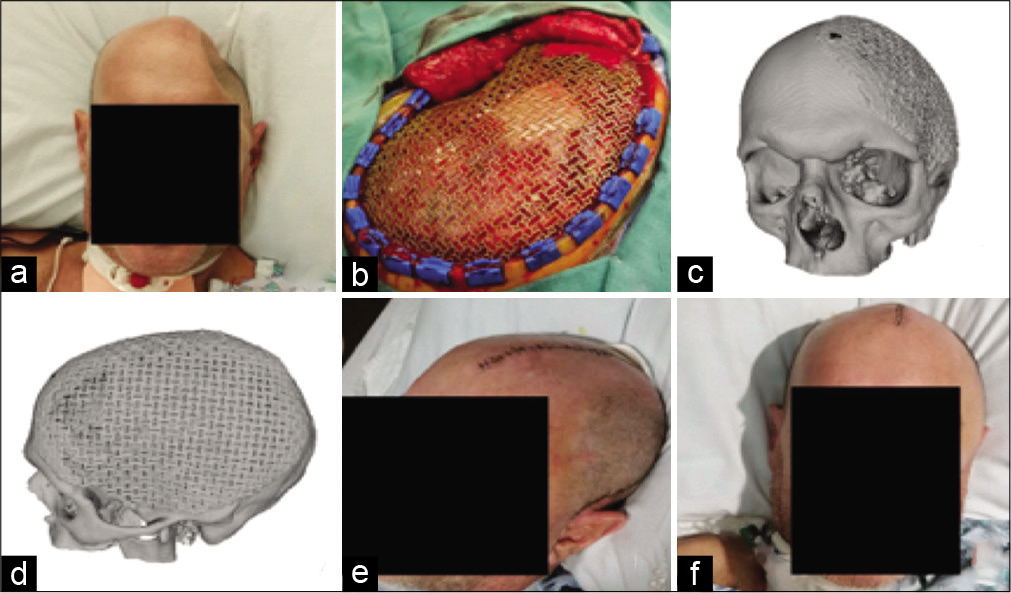
Figure 2:
(a) Preoperative picture shows the large cranial defect. (b) Intraoperative view of premolded titanium mesh spanning cranial defect. (c) Oblique and (d) sagittal views of the 3D reconstruction of postcranioplasty CT scan. (e) Oblique and (f) anterior views of the patient after cranioplasty with excellent cosmetic results.
DISCUSSION
Cranioplasty is a common neurosurgical procedure and is performed for several reasons.
The use of the patient’s bone flap is associated with excellent esthetic results at no additional cost for implants. Under certain circumstances, the use of the patients’ bone flap is not feasible because of mechanical damage, tumor invasion, or infection. In that case, several alternatives are available including a custom PEEK implant, custom titanium mesh, or flat titanium mesh with intraoperative or preoperative molding. Multiple factors dictate the superiority of one option in comparison to others such as cost, cosmetic results, any additional operative time needed, and risk for infection. The flat titanium mesh is cost effective as it costs $5000–8000/ sheet, but it requires intraoperative molding which adds an average of 60 min to the total time of the procedure. This translates into an additional average cost of $4000 as the cost of the use of an operating room in the U.S. averages about $62/min.[
Our technique uses a relatively inexpensive desktop 3D printer to create a patient-specific 3D model used to shape and size the titanium mesh preoperatively with no need for intraoperative molding. The elimination of the intraoperative molding saves about 60 min of intraoperative time which translates into a $4000 cost reduction. Besides, our method results in an implant with superior cosmetic results as its shaped using a model made based on the patient’s precraniectomy CT images. The previous reports have shown that the use of the commercially available custom cranioplasty implants (e.g., PEEK) requires no additional molding time in the operating room, have lower complication rates, and better cosmetic outcomes in comparison to intraoperative molded titanium implants.[
CONCLUSION
The low-cost 3D printing technology allows a timely creation of patient-specific cranial implants with improved esthetic outcomes and significant cost saving. It has the potential to revolutionize the socioeconomic paradigm of medical implants.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aarabi B, Hesdorffer DC, Ahn ES, Aresco C, Scalea TM, Eisenberg HM. Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neurosurg. 2006. 104: 469-79
2. Banks J. Adding value in additive manufacturing: Researchers in the United Kingdom and Europe look to 3D printing for customization. IEEE Pulse. 2013. 4: 22-6
3. Gilardino MS, Karunanayake M, Al-Humsi T, Izadpanah A, Al-Ajmi H, Marcoux J. A comparison and cost analysis of cranioplasty techniques: Autologous bone versus custom computer-generated implants. J Craniofac Surg. 2015. 26: 113-7
4. Gross BC, Erkal JL, Lockwood SY, Chen C, Spence DM. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal Chem. 2014. 86: 3240-53
5. Klein GT, Lu Y, Wang MY. 3D printing and neurosurgery-ready for prime time?. World Neurosurg. 2013. 80: 233-5
6. Lethaus B, Bloebaum M, Koper D, Poort-Ter Laak M, Kessler P. Interval cranioplasty with patient-specific implants and autogenous bone grafts-success and cost analysis. J Craniomaxillofac Surg. 2014. 42: 1948-51
7. Macario A. What does one minute of operating room time cost?. J Clin Anesth. 2010. 22: 233-6
8. Sanan A, Haines SJ. Repairing holes in the head: A history of cranioplasty. Neurosurgery. 1997. 40: 588-603
9. Schubert C, van Langeveld MC, Donoso LA. Innovations in 3D printing: A 3D overview from optics to organs. Br J Ophthalmol. 2014. 98: 159-61
10. Ursan ID, Chiu L, Pierce A. Three-dimensional drug printing: A structured review. J Am Pharm Assoc (2003). 2013. 53: 136-44
11. Walcott BP, Kuklina EV, Nahed BV, George MG, Kahle KT, Simard JM. Craniectomy for malignant cerebral infarction: Prevalence and outcomes in US hospitals. PLoS One. 2011. 6: e29193