- Department of Pediatric Intensive Care Unit, King Abdullah Specialists Children Hospital, Riyadh, Saudi Arabia
- Department of Neurosurgery, King Abdullah Specialists Children Hospital, Riyadh, Saudi Arabia
- Department of General Pediatrics, King Abdullah Specialists Children Hospital, Riyadh, Saudi Arabia
- Department of Pediatric Neuroradiology, King Abdullah Specialists Children Hospital, Riyadh, Saudi Arabia
- Department of Intervention Radiology, King Abdullah Specialists Children Hospital, Riyadh, Saudi Arabia
Correspondence Address:
Rahaf Alanazi, Department of Neurosurgery, King Abdullah Specialists Children Hospital, Riyadh, Saudi Arabia.
DOI:10.25259/SNI_903_2024
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ali Alshehri1, Rahaf Alanazi2, Haifa Bin Dokhi3, Hesham Alshalaan4, Hanan Alqahtani5, Nawaf Alhamied3, Haya Aldabas3, Khalid Althobaiti1, Hamzah Alali1, Naif Alharbi2, Wael Alshaya2. Rare presentation of acute anterior cord syndrome due to fibrocartilaginous embolism in a pediatric patient following minor trauma. 07-Feb-2025;16:34
How to cite this URL: Ali Alshehri1, Rahaf Alanazi2, Haifa Bin Dokhi3, Hesham Alshalaan4, Hanan Alqahtani5, Nawaf Alhamied3, Haya Aldabas3, Khalid Althobaiti1, Hamzah Alali1, Naif Alharbi2, Wael Alshaya2. Rare presentation of acute anterior cord syndrome due to fibrocartilaginous embolism in a pediatric patient following minor trauma. 07-Feb-2025;16:34. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13377
Abstract
Background: Anterior spinal cord syndrome (ASCS) is an extremely rare condition defined as an infarction of the anterior two-thirds of the spinal cord. The type and timing of the clinical presentation, combined with the radiological findings, can provide a focused clinical picture of the severity and outcomes and direct the management plan. We present a rare case report of a pediatric patient with ASCS in acute settings due to a fibrocartilaginous embolism (FCE).
Case Description: We report a 10-year-old girl who was medically and surgically free. She presented with ASCS features 30 min after lifting her younger sister in her back. The clinical presentation consisted of bilateral lower limbs weakness 0/5 according to the medical research council’s scale, weak anal tone, with pain and temperature significantly altered and absent up to T4 level. A diagnosis of anterior spinal infarction due to FCE was made after excluding the possible anterior cord syndrome etiologies. The management consisted of aspirin and extensive physiotherapy, and she significantly recovered over 1 month.
Conclusion: We report a rare case of acute presentation of a pediatric patient with ASCS due to FCE. The timing of a diagnosis affects clinical results. Correlating the radiological findings to the clinical presentation can narrow the differential diagnosis. The literature on the management of these cases is lacking. Animal studies reported a trial of medical therapy.
Keywords: Anterior cord syndrome, Case report, Fibrocartilaginous embolism, Pediatric, Spine trauma
INTRODUCTION
History and definition
Anterior spinal cord syndrome (ASCS) is a rare disorder that accounts for only 8% of all myelopathies and not more than 1% of all strokes. It was first described in 1909 by Spiller in a patient with anterior spinal artery (ASA) thrombosis who was noted to have an infarct at autopsy in the anterior part of the spinal cord, extending from C4 to T3. In 1966, Garland et al.[
CASE PRESENTATION
History of presenting illness
A 10-year-old previously healthy girl presented with acute onset paraplegia and loss of bladder and bowel function, which began approximately 30 min after lifting her. She was playing with her cousin (trying to lift each other on their backs), which led to hyperextension of their back.
Physical examination
Initial examination on admission revealed pupils equal and reactive to light at 2 mm, cranial nerves were intact with everyday speech, language, and visual fields, full strength (5/5) in the upper limbs, complete motor paralysis (0/5) in the lower limbs, decreased sensation to pinprick and temperature “cold” at the level of T4 and below, but intact proprioception and vibrations, and weak anal tone.
Investigations
Urgent unenhanced thoracic and lumbar spine computed tomography (CT) showed L5 bilateral spondylolysis without associated spondylolisthesis. It also revealed a mild posterior disc bulge at the level of L4–L5 L5–S1 without associated neural foraminal narrowing or spinal canal stenosis. These findings did not explain her presentation. Thus, a cervical, thoracic, and lumbar spine magnetic resonance imaging (MRI) [
Figure 1:
(a) SAG T2 SE images of the cervicothoracic spine demonstrate a slit-like high signal along the anterior horn of the spinal cord (white arrow). There is altered disc signal intensity at the T2/3 and T3/4 levels (red arrow). There is a subtle signal at the disc capsule. (b) AX T2 SE images of the cervicothoracic spine demonstrate a high signal along the anterior horn of the spinal cord (white arrow). There is a subtle signal at the disc capsule. (c) AX T2 SE images of the cervicothoracic spine demonstrate a high signal along the anterior horn of the spinal cord. There is a subtle signal at the disc capsule (yellow arrow). SAG: Sagittal,SE: Spin echo, AX: axial, T2 SE: T2-Weighted
Figure 2:
(a) SAG T2 SE images of the cervicothoracic spine demonstrate worsening cord anterior horn edema (white arrow). The vertebral altered signal is more evident suggestive of contusion (red arrow). (b) SAG T1 SE post gadolinium administration images of the cervicothoracic spine demonstrate heterogeneous anterior cord enhancement consistent with post ischemic re-perfusion (white arrow), (c) SAG ADC images of the cervicothoracic spine demonstrate restricted diffusion of the anterior cord consistent with ischemia (yellow arrow), (d) time-resolved – magnetic resonance angiography (MRA) sequences TRICKS 3T GE system in arterial phase demonstrate nonvisualization of normal anterior spinal artery (ASA) in expected anatomic location (white arrow), (e) time-resolved-MRA sequences TRICKS 3T GE system in early venous phase with reconstructed axial multiplanar reformation (MPR) demonstrate non-visualization of normal ASA in expected anatomic location and contrast pool at left neural foramina consistent with congested venous plexus (yellow arrow), and (f) time-resolved-MRA sequences TRICKS 3T GE system in early venous phase with reconstructed axial MPR demonstrate non-visualization of normal anterior spinal artery (ASA) in expected anatomic location and contrast pool at left neural foramina consistent with congested venous plexus (yellow arrow). SAG: Sagittal, T2 SE: T2-Weighted spin echo, ADC: Apparent diffusion coefficient, MRA: Magnetic Resonance Angiography, TRICKS: Time-Resolved imaging of contrast kinetics, GE: Gradient echo
Hospital course
No underlying abnormal arteriovenous malformation or aneurysm was observed. Spinal digital subtraction angiography [
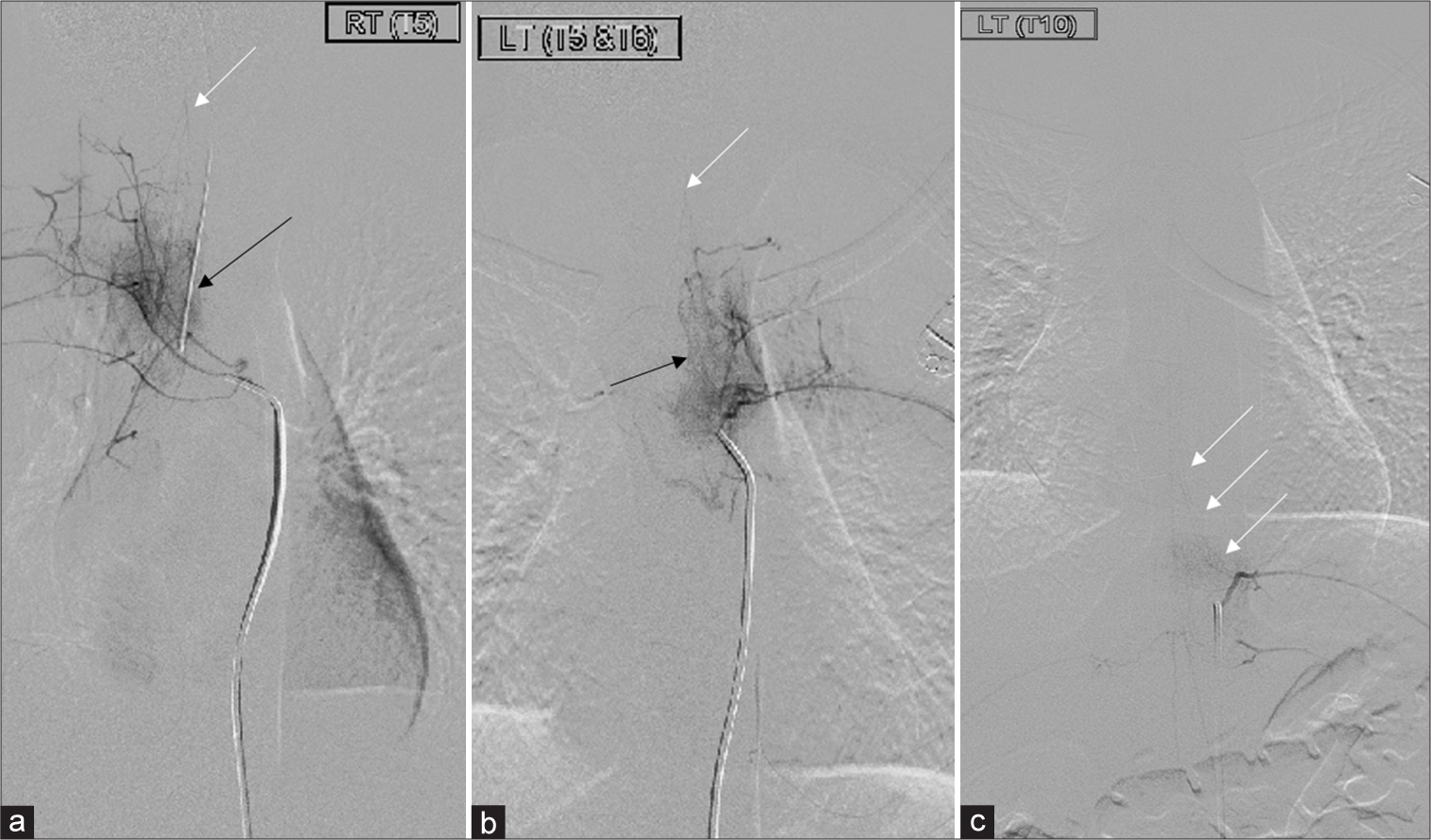
Figure 3:
(a) Selected digital subtracted spinal angiogram images at T5–T6 level showed vertebral body hyperemia related to the trauma (black arrows). Neither vascular injury nor shunting was noted. A faint flow was seen at the upper thoracic T1 could represent the spinal artery (white arrows). However, at the targeted area T5–T6, there was absent flow (b) Selected digital subtracted spinal angiogram images at T5–T6 level showed vertebral body hyperemia related to the trauma (black arrows). Neither vascular injury nor shunting was noted. A faint flow was seen at the upper thoracic T1 could represent the spinal artery (white arrows). However, at the targeted area T5–T6, there was absent flow. (c) The artery of Adamkiewicz is seen at the left T10 level (white arrows).
Follow-up after 1 month
At a follow-up after 3 weeks, the patient surprisingly ambulated with lower limb power 4 out of 5.
DISCUSSION ASCS
Incidence and prevalence
ASCS is an infrequent neurologic condition, accounting for approximately 1.2% of all vascular neurological disorders. In the review by Foo and Rossier,[
ASCS characteristics
It is generally described as motor deficits below the level of injury and impairment of pain and temperature while preserving vibration and proprioception sensation. By understanding the anatomy, the clinical presentation can point to the area of injury.
ASA origin
The ASA forms from the bilateral vertebral arteries at the foramen magnum and runs as an uninterrupted artery within the anterior median sulcus of the spinal cord to the conus medullaris. Radicular arteries enter the spinal canal through the intervertebral foramen and primarily supply the nerve roots; however, some anastomoses contribute to the ASA. The largest of these radicular arteries is the artery of Adamkiewicz, also known as arteria radicularis magna, which most commonly arises from a left intercostal artery between segments T9 and T12 but can vary anatomically. The ASA branches into small sulcal and penetrating arteries that enter the body of the spinal cord.[
ASA branches and supply
The ASA supplies blood to the spinal cord’s bilateral anterior and lateral horns and the bilateral spinothalamic and corticospinal tracts. The anterior horns and corticospinal tracts control the somatic motor system from the neck to the feet. The lateral horns, spanning levels T1 to L2 of the spinal cord, comprise the neuronal cell bodies of the sympathetic nervous system. The spinothalamic tracts relay pain, temperature, and sensory information.[
ASCS evaluation
History and physical examinations are essential for timing diagnosis. When the patient presents with symptoms and signs similar to those described above, ruling out vascular causes could be the top priority for establishing the differential diagnosis. CT angiography can be done in patients with abdominal pain and hypotension to rule out aortic dissection; CT spine can be used when suspecting spine trauma or vertebral artery injury. MRI is the gold standard with the following sequencing: T2-weighted (T2W) sagittal, T2W axial, short-tau inversion recovery sagittal, diffuse weighted images (DWI), and contrast-enhanced T1W sagittal sequences.[
ASCS management
The management is mainly supportive, limiting underlying causes and preventing future complications. Steroids can be given in cases of edema. Some studies have described the benefit of lumbar drain, increasing MAP from 60 to 90 or 100 mmHg.[
ASCS outcomes and prognosis
The reported etiologies consist of idiopathic with the best motor recovery rates, with 92.9% of these patients regaining some motor function. ASCS caused by post-infectious myelopathy or vaccination had an 88.9% recovery rate, while cases due to ASA occlusion and aortic lesions had lower recovery rates of 33.3% and 20%, respectively. The data highlight the variable outcomes based on the underlying cause of the syndrome.[
Mortality during the hospital stay was 22.2%, and most survivors had significant long-term disabilities, with 57.1% requiring a wheelchair.[
Fibrocartilaginous embolism (FCS) history
Although FCE has been widely reported among animals, especially dogs, it is a rare cause of human anterior cord infarction.[
FCS incidence and prevalence
Since 1961, only 25 cases of ACS secondary to FCE have been reported in children aged <18 years old, with female predominance (16 female vs. nine male).[
Diagnosing FCS as a cause of ASCS
Such cases are infrequently experienced in pediatric emergency departments. Therefore, confirming the diagnosis is challenging as it resembles other conditions with ascending paralysis, such as Guillain–Barre syndrome, transverse myelitis, and other neurological diseases.[
CONCLUSION
This case report highlights the rare presentation of ASCS due to FCS in a pediatric patient following hyperextension of the back. Our management plan was initiated with a spinal cord injury protocol that includes inotropic support targeting a MAP of 80 and above to prevent further spinal cord injury. She was also started on antiplatelet therapy and extensive physiotherapy. Favorable outcomes were achieved during 1 month of follow-up. Limited data from human studies are published in the literature. Further studies are needed to support the appropriate and timing of medical management for these cases.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. AbdelRazek MA, Mowla A, Farooq S, Silvestri N, Sawyer R, Wolfe G. Fibrocartilaginous embolism: A comprehensive review of an under-studied cause of spinal cord infarction and proposed diagnostic criteria. J Spinal Cord Med. 2016. 39: 146-54
2. AbdelRazek M, Elsadek R, Elsadek L. Case series of two patients with fibrocartilaginous embolism mimicking transverse myelitis of the spinal cord. J Clin Neurosci. 2017. 40: 66-8
3. Bansal S, Brown W, Dayal A, Carpenter JL. Posterior spinal cord infarction due to fibrocartilaginous embolization in a 16-year-old athlete. Pediatrics. 2014. 134: e289-92
4. Cuello JP, Ortega-Gutierrez S, Linares G, Agarwal S, Cunningham A, Mohr JP. Acute cervical myelopathy due to presumed fibrocartilaginous embolism: A case report and systematic literature review. J Spinal Disord Tech. 2014. 27: E276-81
5. Duprez TP, Danvoye L, Hernalsteen D, Cosnard G, Sindic GJ, Godfraind C. Fibrocartilaginous embolization to the spinal cord: Serial MR imaging monitoring and pathologic study. AJNR Am J Neuroradiol. 2005. 26: 496-501
6. Foo D, Rossier A. Anterior spinal artery syndrome and its natural history. Spinal Cord. 1983. 21: 1-10
7. Garland H, Greenberg J, Harriman DG. Infarction of the spinal cord. Brain. 1966. 89: 645-62
8. Han JJ, Massagli TL, JaCe KM. Fibrocartilaginous embolism-an uncommon cause of spinal cord infarction: A case report and review of the literature. Arch Phys Med Rehabil. 2004. 85: 153-7
9. Jones DD, Watson RE, Heaton HA. Presentation and medical management of Fibrocartilaginous embolism in the emergency Department. J Emerg Med. 2016. 51: 315-8
10. Klakeel M, Thompson J, Srinivasan R, McDonald F. Anterior spinal cord syndrome of unknown etiology. Proc (Bayl Univ Med Cent). 2015. 28: 85-7
11. Mosberg WH, Voris HC, Duffy J. Paraplegia as a complication of sympathectomy for hypertension. Ann Surg. 1954. 139: 330-4
12. Nakstad I, Randjelovic I, Bergan H, Evensen K. Fibrocartilaginous embolism as a cause of anterior spinal artery syndrome?. Tidsskr Nor Laegeforen. 2020. 140:
13. Naiman JL, Donohue WL, Prichard JS. Fatal nucleus pulposus embolism of spinal cord after trauma. Neurology. 1961. 11: 83-e7
14. Quesney G, Lefaucheur R, Hebant B. Clinically suspected concomitant spinal cord and vertebrobasilar infarctions caused by fibrocartilaginous embolism. J Clin Neurosci. 2020. 77: 222-4
15. Ryken TC, Hurlbert RJ, Hadley MN, Aarabi B, Dhall SS, Gelb DE. The acute cardiopulmonary management of patients with cervical spinal cord injuries. Neurosurgery. 2013. 72: 84-92
16. Salvador de la Barrera S, Barca-Buyo A, Montoto-Marqués A, Ferreiro-Velasco ME, Cidoncha-Dans M, Rodriguez-Sotillo A. Spinal cord infarction: prognosis and recovery in a series of 36 patients. Spinal Cord. 2001. 39: 520-5
17. Strohm TA, John S, Hussain MS. Cerebrospinal fluid drainage and blood pressure elevation to treat acute spinal cord infarct. Surg Neurol Int. 2018. 9: 195
18. Tosi L, Rigoli G, Beltramello A. Fibrocartilaginous embolism of the spinal cord: A clinical and pathogenetic reconsideration. J Neurol Neurosurg Psychiatry. 1996. 60: 55-60
19. Weisman AD, Adams RD. The neurological complications of dissecting aortic aneurysm. Brain. 1944. 67: 69-92
20. Yadav N, Pendharkar H, Kulkarni GB. Spinal cord infarction: Clinical and radiological features. J Stroke Cerebrovasc Dis. 2018. 27: 2810-21
21. Yamaguchi H, Nagase H, Nishiyama M, Tokumoto S, Toyoshima D, Akasaka Y. Fibrocartilaginous embolism of the spinal cord in children: A case report and review of literature. Pediatr Neurol. 2019. 99: 3-6
22. Yogendranathan N, Herath HM, Jayamali WD, Matthias AT, Pallewatte A, Kulatunga A. A case of anterior spinal cord syndrome in a patient with unruptured thoracic aortic aneurysm with a mural thrombus. BMC Cardiovasc Disord. 2018. 18: 48