- Department of Anesthesiology, Advocate Illinois Masonic Medical Center, Chicago, United States.
- Ghaly Neurosurgical Associates, Aurora, United States.
- Department of Anesthesiology, University of Illinois, Chicago, Illinois, United States.
Correspondence Address:
Ramsis F. Ghaly
Department of Anesthesiology, Advocate Illinois Masonic Medical Center, Chicago, United States.
Department of Anesthesiology, University of Illinois, Chicago, Illinois, United States.
DOI:10.25259/SNI_65_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ramsis F. Ghaly, Armen Haroutanian, Parnia Khamooshi, Jessica Patricoski, Kenneth D. Candido, Nebojsa Nick Knezevic. Recent decline in the use of invasive neurocritical care monitoring for traumatic brain injury: A case report. 25-Apr-2020;11:85
How to cite this URL: Ramsis F. Ghaly, Armen Haroutanian, Parnia Khamooshi, Jessica Patricoski, Kenneth D. Candido, Nebojsa Nick Knezevic. Recent decline in the use of invasive neurocritical care monitoring for traumatic brain injury: A case report. 25-Apr-2020;11:85. Available from: https://surgicalneurologyint.com/surgicalint-articles/9980/
Abstract
Background: In this article, we discuss the dramatic decline in the utilization of invasive cranial monitoring of patients with traumatic brain injury (TBI).
Case Description: A 52-year-old male presented with a severe TBI following a motor vehicle accident. The initial computed tomography scan showed a subdural hematoma, and the patient underwent a craniotomy. However, preoperatively, intraoperatively, and postoperatively, the critical care team never utilized invasive cranial monitoring. Therefore, when the patient expired several weeks later due to multiorgan failure, his death was in part attributed to the neurocritical care specialists’ failure to employ invasive cranial monitoring techniques.
Conclusion: Evidence-based and defensive medicine, cost containment, and a lack of leadership have contributed to neurocritical care specialists’ increased failure to utilize invasive hemodynamic and neurological monitoring for TBI.
Keywords: Brain trauma foundation, Decline invasive monitoring, Intracranial pressure, Neurocritical monitoring, Traumatic brain injury
INTRODUCTION
Traumatic brain injury (TBI) causes significant morbidity and mortality in the United States; 1.7 million TBIs account for 275,000 hospitalizations and 52,000 deaths annually.
Over the past few decades, patients with TBI were connected to various invasive hemodynamic and cerebral monitors including central venous pressure, pulmonary capillary wedge pressure, pulmonary artery catheter, electroencephalography, transcranial Doppler (TCD), ideal hemoglobin, brain tissue oxygen monitoring, and sensory evoked potentials.[
However, despite these “benefits,” many centers are failing to utilize continuous invasive hemodynamic and cerebral monitoring critical care settings to maximize recovery from TBI. Guidelines are now primarily comprised of review articles and evidence-based practices with a lack of published evidence with respect to the Brain Trauma Foundation (BTF) guidelines.[
CASE DESCRIPTION
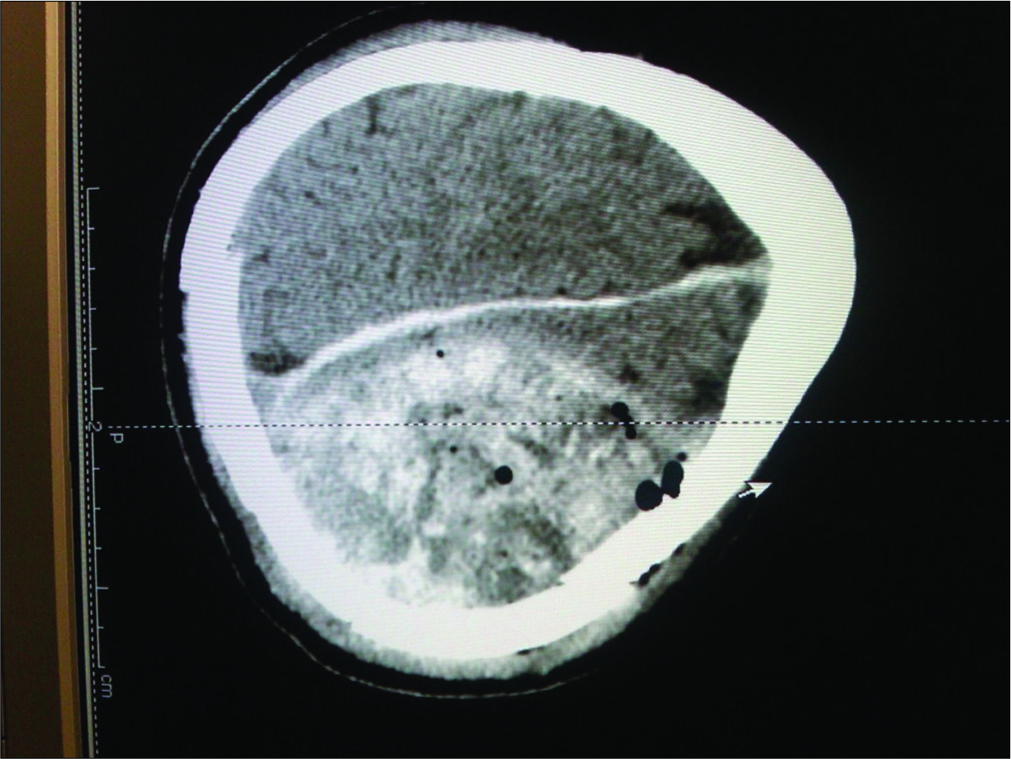
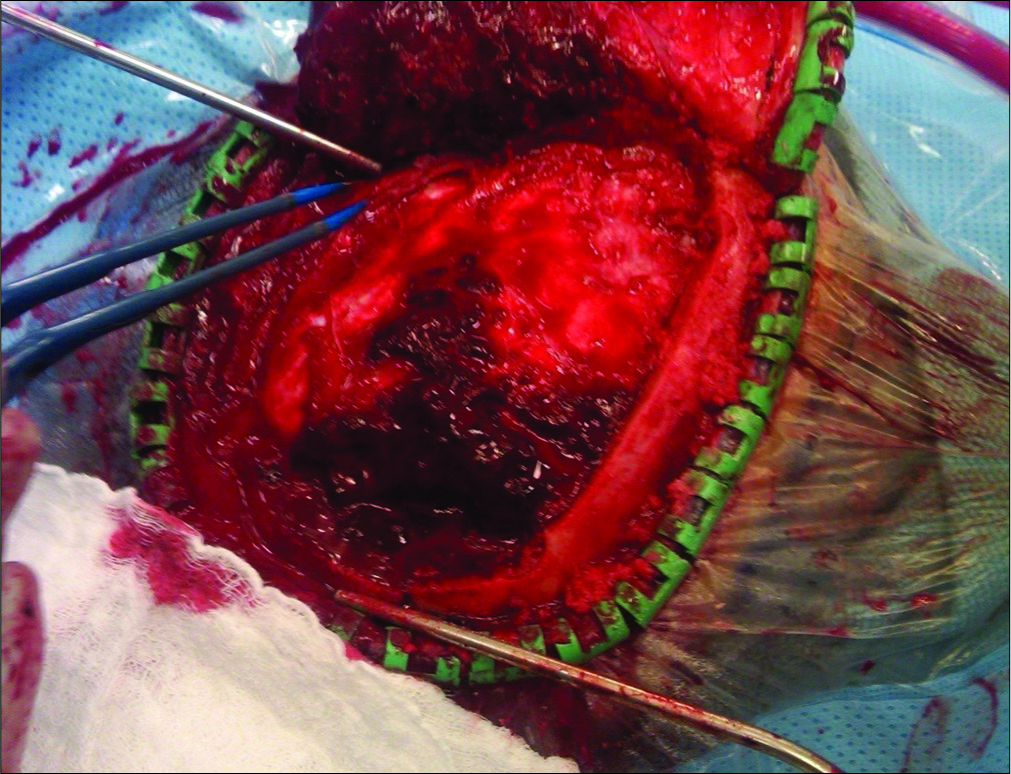
A 52-year-old Caucasian male sustained a TBI due to a motor vehicle accident. He had a Glasgow Coma Scale (GCS) of 13; his mean arterial pressure of 60 mmHg was maintained by a norepinephrine drip. When he developed a dilated left pupil with decerebrate posturing (GCS 5), he required intubation. The computed tomography (CT) showed a large left epidural hematoma (EDH) with a 2 cm midline shift warranting a craniotomy [
Postoperatively, in the neurocritical invasive care unit, standard ASA monitoring included oxygen saturation (pulse oximeter), end-tidal CO2 monitoring, standard electrocardiographic, arterial line monitoring of blood pressure (BP), and serial arterial blood gas assessments were performed.
The postoperative CT scans showed continued resolution of the EDH and improvement of the edema and midline shift (GCS of 6–7). However, at the end of the 2nd week, the patient expired due to complications of TBI (e.g., multiorgan failure). The main question is whether the use of an ICP monitor would have changed this patient’s course and outcome.
DISCUSSION
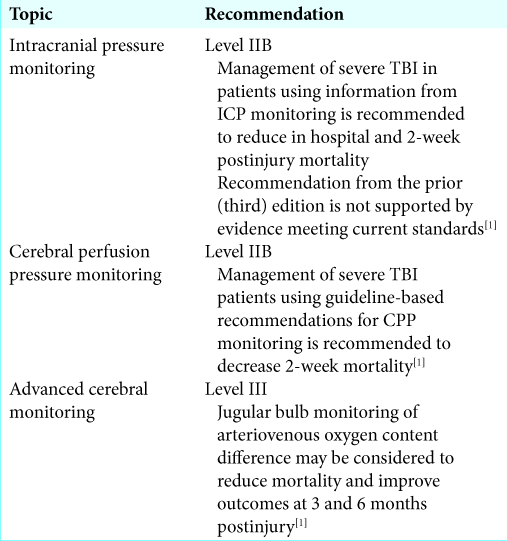
The BTF most recently cited three parameters for management guidelines; CPP, ICP, and jugular bulb monitoring of arteriovenous oxygen content difference [
ICP monitoring
The BTF recommends that “ICP should be monitored in all salvageable patients with a severe TBI and an abnormal CT scan.” Furthermore, ICP monitoring is indicated in patients with severe TBI with a normal CT scan if two or more of the following features are noted at admission: age over 40 years, unilateral or bilateral motor posturing, or systolic BP <90 mmHg. A meta-analysis study by Stein et al. showed 12% decrease in mortality and 6% increase in favorable outcomes in patients with ICP monitors.[
Jugular bulb venous saturation monitoring
The jugular venous oxygen saturation (SjvO2) is an indicator of both cerebral oxygenation and cerebral metabolism.
There have been improvements in outcome by utilizing this method along with ICP/CPP monitoring in comparison with traditional methods alone.[
Brain tissue oxygen tension
Brain tissue oxygenation (Pti02) is a modality used to measure bedside focal brain oxygenation using a microcatheter inserted in the frontal white matter. Studies have demonstrated favorable outcomes in patients with combined ICP/CPP and Pti02-guided therapy.[
Microdialysis monitoring of extracellular glutamate
Several studies have implied a key role of glutamate, an excitatory amino acid, in the pathophysiology of a TBI.[
Cerebral autoregulation monitoring with TCD
TCD measures systolic, mean, and diastolic cerebral blood flow (CBF) velocities and calculates the pulpability index from basal intracranial arteries and can be useful in patients with severe TBI to detect low CBF.
Analysis of invasive neuromonitoring techniques
The vicious cycle ensues in which the brain is left unmonitored due to evidence-based practices using outcome studies, despite articles demonstrating lack of randomization and hindsight bias (sicker patients were more likely to receive the invasive monitoring).[
Shift away from invasive monitoring
Several ideas may be postulated regarding the possible causes of shifting away from invasive monitoring and the lack of global protocols: (1) defensive medicine; (2) cost- effectiveness; (3) lack of leadership; and (4) limited human resources. Why should a diagnostic test be limited to only addressing mortality reduction, and not toward the accurate detection of the diagnoses, and institution of optimal treatment? Certainly, as in this case, less monitoring does not lead to better outcomes.
CONCLUSION
For treating TBI, multidisciplinary efforts using multimodal approaches should be pursued.[
Authors’ contributions
All authors have participated in the preparation of the manuscript and concur with the findings and results.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017. 80: 6-15
2. Faul M, Wald MM, Rutland-Brown W, Sullivent EE, Sattin RW. Using a cost-benefit analysis to estimate outcomes of a clinical treatment guideline: Testing the brain trauma foundation guidelines for the treatment of severe traumatic brain injury. J Trauma. 2007. 63: 1271-8
3. Haddad SH, Arabi YM. Critical care management of severe traumatic brain injury in adults. Scand J Trauma Resusc Emerg Med. 2012. 20: 12-
4. Lazaridis C, Robertson CS. The role of multimodal invasive monitoring in acute traumatic brain injury. Neurosurg Clin N Am. 2016. 27: 509-17
5. Roh D, Park S. Brain multimodality monitoring: Updated perspectives. Curr Neurol Neurosci Rep. 2016. 16: 56-
6. Stein SC, Georgoff P, Meghan S, Mirza KL, El Falaky OM. Relationship of aggressive monitoring and treatment to improved outcomes in severe traumatic brain injury. J Neurosurg. 2010. 112: 1105-12
7. Stiefel MF, Spiotta A, Gracias VH, Garuffe AM, Guillamondegui O, Maloney-Wilensky E. Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J Neurosurg. 2005. 103: 805-11
8. Stocchetti N, Le Roux P, Vespa P, Oddo M, Citerio G, Andrews PJ. Clinical review: Neuromonitoring-an update. Crit Care. 2013. 17: 201-
9. Stone JL, Ghaly RF, Hughes JR. Evoked potentials in head injury and states of increased intracranial pressure. J Clin Neurophysiol. 1988. 5: 135-60