- Department of Neurosurgery, Hospital Força Aérea do Galeão, Rio de Janeiro, Brazil,
- Department of Neurosurgery, Atenas Medical School, Passos, Minas Gerais, Brazil,
- Department of Neurosurgery, Hospital Italiano de Buenos Aires, Buenos Aires, Argentina.
Correspondence Address:
Dan Zimelewicz Oberman
Department of Neurosurgery, Hospital Italiano de Buenos Aires, Buenos Aires, Argentina.
DOI:10.25259/SNI_509_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Dan Zimelewicz Oberman1, Nícollas Nunes Rabelo2, Jorge Luiz Amorim Correa1, Pablo Ajler3. Relationship of superior sagittal sinus with sagittal midline: A surgical application. 25-Sep-2020;11:309
How to cite this URL: Dan Zimelewicz Oberman1, Nícollas Nunes Rabelo2, Jorge Luiz Amorim Correa1, Pablo Ajler3. Relationship of superior sagittal sinus with sagittal midline: A surgical application. 25-Sep-2020;11:309. Available from: https://surgicalneurologyint.com/surgicalint-articles/10284/
Abstract
Background: Interhemispheric approach is widely used to surgical management of midline tumors and vascular lesion in and around the third ventricle. Complete exposure of the superior sagittal sinus to obtain adequate working space of midline lesion is difficult, because of the risk to inadvertent injury to the sinus and bridging veins, which may cause several neurological deficits. Understanding the SSS neuroanatomy and its relationships with external surgical landmarks avoid such complications. The objective of this study is to accurately describe the position of SSS and its displacement in relation with sagittal midline by magnetic resonance imaging.
Methods: A retrospective cross-sectional, observational study was performed. Magnetic resonance image of 76 adult patients with no pathological imaging was analyzed. The position of the halfway between nasion and bregma, bregma, halfway between bregma and lambda, and lambda was performed. The width and the displacement of the superior sagittal sinus accordingly to the sagittal midline were assessed in those landmarks.
Results: The mean width of superior sagittal sinus at halfway between nasion and bregma, bregma, halfway between bregma and lambda, and lambda was 5.62 ± 2.5, 6.5 ± 2.8, 7.4 ± 3.2, and 8.5 ± 2.1 mm, respectively, without gender discrepancy. The mean displacement according to the midline at those landmarks showed a statistically significant difference to the right side among sexes.
Conclusion: In this study, we demonstrate that sagittal midline may approximate external location of the superior sagittal sinus. Our data showed that in the majority of the cases, the superior sagittal sinus is displaced to the right side of sagittal midline as far as 16.3 mm. The data we obtained provide useful information that suggest that neurosurgeons should use safety margin to perform burr holes and drillings at the sagittal midline.
Keywords: Anatomical study, Bregma, Coronal suture, Dural sinus, Superior sagittal sinus
INTRODUCTION
Interhemispheric approach is widely used for surgical management of midline tumors and vascular lesions in and around the third ventricle.[
In general, the superior sagittal suture has been used to approximate the location of SSS, which is considered an external landmark of SSS between the bregma and the lambda, and it is still being used in neurosurgical practices. However, this external anatomical landmark is not useful anteriorly to the bregma, and previous studies, showed that it is not accurate to predict the location of the SSS.[
Complete exposure of the SSS to obtain adequate working space of midline lesion is difficult because of the risk of inadvertent injury to the sinus and bridging veins, which may cause several neurological deficits.[
Although there are some reports about the anatomy of SSS and its relationship with sagittal suture, most of them were performed on cadaveric specimens with relatively low number of cases, and further studies are needed. Our aim is to accurately describe the position of SSS and its displacement in relation with sagittal midline, but unlike the previous reports, to measure them on a wide quantity of samples by magnetic resonance imaging.
MATERIALS AND METHODS
Patients
Magnetic resonance imaging (MRI) examinations were retrieved from our electronic medical records. The study included adult patients who consulted the health care of our hospital between March 2018 and February 2020 and who had no pathological imaging findings.
Anatomical definitions
The width of the superior sagittal sinus from different sagittal midline convexity craniometric points was performed: (Na-Br) halfway between the nasion and bregma, Br in the bregma, Br-Lamb halfway between the bregma and lambda, and Lamb in the lambda. Subsequently, we measure the displacement of the SSS to the right and to the left accordingly to the sagittal midline in those landmarks. We also evaluated the dominant transverse sinus that primarily drains the SSS [
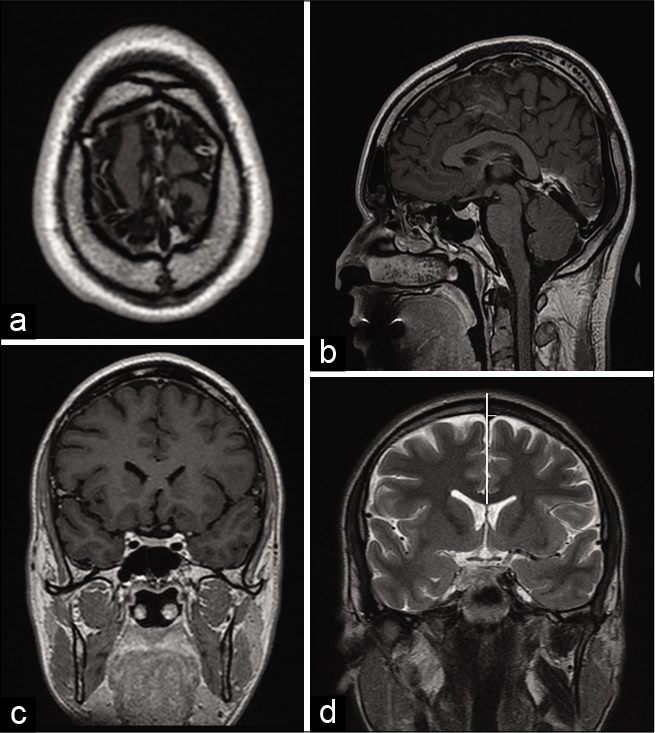
Figure 1:
Example of location in (a) axial, sagittal (b), and coronal (c) plane of magnetic resonance image and measurement at bregma in coronal magnetic resonance image in T2 sequence (d). Vertical white line is placed at the sagittal midline. Distance from the sagittal midline to the right and left edge of superior sagittal sinus. This image shows the displacement of the superior sagittal sinus toward the left at bregma. The bregma is the hypointense gap at the top of the head (d).
The anatomical key points were defined as shown below:
Nasion: Intersection point between frontal and nasal bones, located in a depressed area that connects the nasal bridge and the glabella. Bregma: Intersection point between frontal and sagittal sutures. It was first located in the upper axial planes of the skull, to accurately identificate it in the sagittal plane. It appears as a hypointense line that interrupts the full-thickness continuity of the hyperintense bony signal. Lambda: Intersection point between lambdoid and sagittal sutures. It was first located in the upper axial planes of the skull, to accurately identificate it in the sagittal plane. It appears as a hypointense line that interrupts the full-thickness continuity of the hyperintense bony signal.
Magnetic resonance imaging data acquisition and image processing
Coronal T2 MRI was acquired using a 1.5-T MR imaging unit (Signa®; General Electric Medical Systems) with a multislice scanning acquisition protocol. Intravenous contrast administration was not required. The images were processed and analyzed by the software OsiriX MD image program, version 11.0 in axial, coronal, and sagittal planes for the following points: Na-Br, Br, Br-Lamb, and Lamb.
Statistical analysis
An observational retrospective cross-sectional study was made. All analyses were performed using the Statistical Software Package (Stata for Macbook version 13.0). The Shapiro–Wilk test was used to test the normality assumption. For the interobserver reproducibility analysis, we used the intraclass correlation coefficient (ICC). Relative frequencies were calculated for categorical data. Continuous data were expressed as mean ± standard deviation (SD). The two-tailed independent sample t-test was performed to compare means between groups. P <0.05 was considered to be statistically significant.
RESULTS
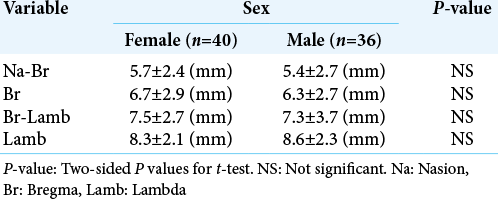
During this period, 76 patients were included in this study. Forty (52.6%) were female and 36 (47.4%) were male [
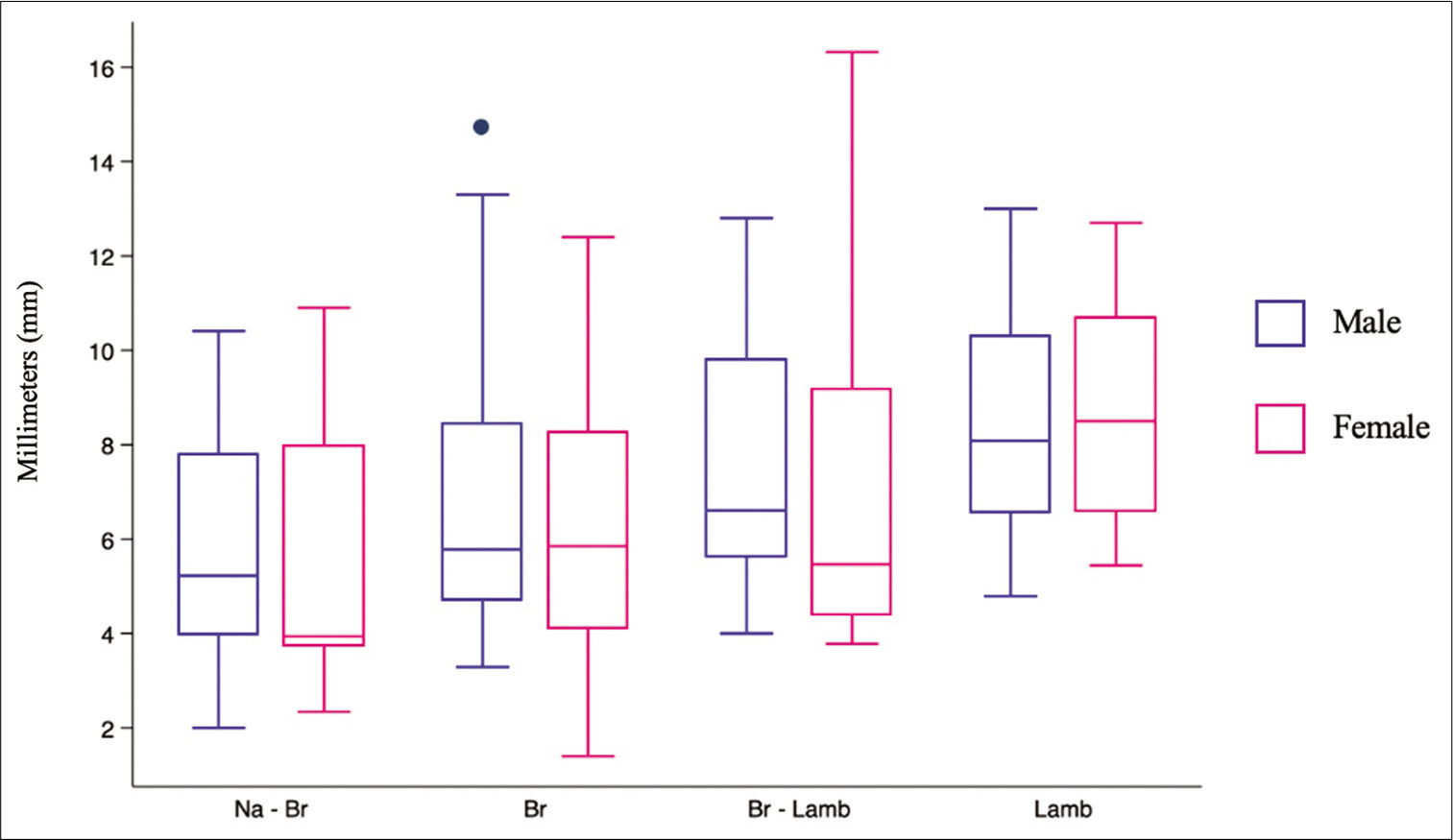
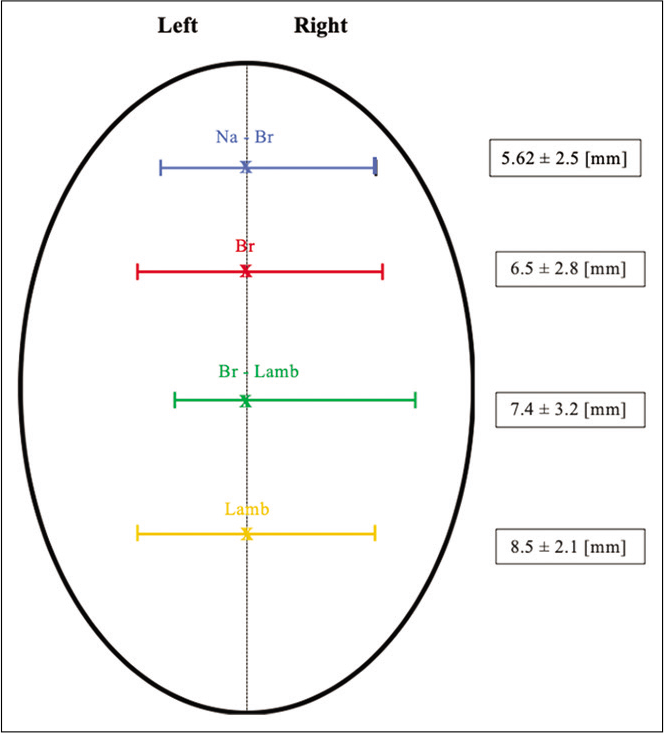
The width of the SSS ranged from 2 to 10.9 mm in the halfway between nasion and bregma (Na-Br) (mean 5.62 ± 2.5 mm), from 1.4 to 13.4 mm in the bregma (Br) (mean 6.5 ± 2.8 mm), from 3.78 to 16.32 mm in the halfway between bregma and lambda (Br-Lamb) (7.4 ± 3.2 mm), and from 4.79 to 13 mm in lambda (Lamb) (mean 8.5 ± 2.1 mm) [
There were no significant differences in the mean width of SSS measurements among both sexes [
We also made observations and measurements concerning the alignment between midline and deviation of the SSS [
Figure 3:
Scheme representing the width of sinus at the four landmark points and the range of its deviation. SSS at halfway between nasion-bregma (blue), bregma (red), bregma-lambda (green), and lambda (orange). Br: Bregma; Br-Lamb: Midpoint bregma-lambda, Lam: Lambda; N: Nasion, Na-Br: Midpoint nasion-bregma, SSS: Superior sagittal sinus.
The comparison between male and female showed a statistical significant difference when the measurement was related to the displacements of SSS according to the midline in the following measures (Na-Br, Br, and Lamb), but was not when related to Br-Lamb. These results are shown in [
Finally, we determined which transverse sinus primarily drained the SSS. A dominant drain was present in 17 (22.36%) patients, of which 12 (70.5%) drained to the right sided.
There was an excellent intraclass correlation coefficient between the two observer measurements of the Na-Br, Br, Br-Lamb, and Lamb with an average k value of 0.82, 0.92, 0.97, and 0.89, respectively.
DISCUSSION
The SSS is the longest dural sinus, which courses in the midline along the superior edge of the falx cerebri, beginning just behind the frontal bone and grows larger as it continues posteriorly, as it receives blood from the frontal, parietal, and occipital superior cerebral veins and the diploic veins.[
Intracranial venous structures have gained especial neurosurgical interest due to improvements in neuroimaging techniques and clinical applications.[
The SSS is often encountered during neurosurgical procedures for interhemispheric transcallosal approaches, among many other access points into the skull. Exposure of this dural venous sinus is often required in the management of lesions situated in proximity to the falx and tentorium. Although extremely useful, the navigation systems are not available in many countries around the world. Current location of the SSS on the external cranial surface for proper positioning of supratentorial craniotomy and for transoperative orientation is still mostly based in cranial topographic anatomy studies, performed especially in cadaveric specimens. Although the sagittal suture can be used as a rough external landmark to estimate placement of the underlying SSS, it is not an accurate landmark[
We found that SSS width in these different landmarks (Na-Br, Br, Br-Lamb, and Lamb) was 5.62 ± 2.5, 6.5 ± 2.8, 7.4 ± 3.2, and 8.5 ± 2.1 mm, respectively. Tubbs et al and Samadian[
Reis et al.[
CONCLUSION
For neurosurgical approaches to the midline, neurosurgeons should be aware that sagittal midline and sagittal suture are not reliable landmarks for the SSS course. However, it can approximate the external location of the SSS. Our data, in accordance with the previous published study, confirm that SSS is in the majority of the cases displaced to the right side of the sagittal midline, as far as 16.3 mm. Thus, we suggest that neurosurgeons should use 1.7 cm as safety margin to perform burr holes and drillings. Always when possible neurosurgeon should carefully analyze preoperative imaging and consider SSS location and relationship with external landmarks.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
References
1. Alvernia JE, Lanzino G, Melgar M, Sindou MP, Mertens P. Is exposure of the superior sagittal sinus necessary in the interhemispheric approach?. Neurosurgery. 2009. 65: 962-4
2. Aryan HE, Ozgur BM, Jandial R, Levy ML. Complications of interhemispheric transcallosal approach in children: Review of 15 years experience. Clin Neurol Neurosurg. 2006. 108: 790-3
3. Brockmann C, Kunze S, Scharf J. Computed tomographic angiography of the superior sagittal sinus and bridging veins. Surg Radiol Anat. 2011. 33: 129-34
4. Brockmann C, Kunze SC, Schmiedek P, Groden C, Scharf J. Variations of the superior sagittal sinus and bridging veins in human dissections and computed tomography venography. Clin Imaging. 2012. 36: 85-9
5. Bruno-Mascarenhas MA, Ramesh VG, Venkatraman S, Mahendran JV, Sundaram S. Microsurgical anatomy of the superior sagittal sinus and draining veins. Neurol India. 2017. 65: 794-800
6. Campero A, Ajler P, Emmerich J, Goldschmidt E, Martins C, Rhoton A. Brain sulci and gyri: A practical anatomical review. J Clin Neurosci. 2014. 21: 2219-25
7. Cervante TP, Arnaud E, Brunelle F, di Rocco F. Unilateral coronal synostosis: Can we trust the sagittal suture as a landmark for the underlying superior sagittal sinus?. J Neurosurg Pediatr. 2016. 17: 589-94
8. Chi JH, Lawton MT. Posterior interhemispheric approach: Surgical technique, application to vascular lesions, and benefits of gravity retraction. Neurosurgery. 2006. 59: ONS41-9
9. Cho YH, Yang IC, Kim YS, Kim TS, Joo SP. Bifrontal interhemispheric approach involving cutting the superior sagittal sinus for distal anterior cerebral artery aneurysms. World Neurosurg. 2019. 127: e1057-63
10. DiMeco F, Li KW, Casali C, Ciceri E, Giombini S, Filippini G. Meningiomas invading the superior sagittal sinus: Surgical experience in 108 cases. Neurosurgery. 2008. 62: 1124-35
11. Gordon WE, Michael LM, van Landingham MA. Exposure of dural venous sinuses: A review of techniques and description of a single-piece troughed craniotomy. Cureus. 2018. 10: e2184
12. Han H, Tao W, Zhang M. The dural entrance of cerebral bridging veins into the superior sagittal sinus: An anatomical comparison between cadavers and digital subtraction angiography. Neuroradiology. 2007. 49: 169-75
13. Kubota M, Saeki N, Yamaura A, Ono J, Ozawa Y. Influences of venous involvement on postoperative brain damage following the anterior interhemispheric approach. Acta Neurochir. 2001. 143: 321-6
14. Lawton MT, Golfinos JG, Spetzler RF. The contralateral transcallosal approach: Experience with 32 patients. Neurosurgery. 1996. 39: 729-34
15. Mortazavi MM, Denning M, Yalcin B, Shoja MM, Loukas M, Tubbs RS. The intracranial bridging veins: A comprehensive review of their history, anatomy, histology, pathology, and neurosurgical implications. Childs Nerv Syst. 2013. 29: 1073-8
16. Oberman DZ, Rasmussen J, Toscano M, Goldschmidt E, Ajler P. Computed tomographic localization of the central sulcus: A morphometric study in adult patients. Turk Neurosurg. 2017. 28: 877-88
17. Ohara K, Inoue T, Ono H, Kiyofuji S, Tamura A, Saito I. Technique for rerouting a bridging vein that hinders the anterior interhemispheric approach: A technical note. Acta Neurochir (Wien). 2017. 159: 1913-8
18. Oliveira MF, Teixeira MJ, Norremose KA, Matushita H, Oliveira ML, Shu EB. Surgical technique of retrograde ventricle-sinus shunt is an option for the treatment of hydrocephalus in infants after surgical repair of myelomeningocele. Arq Neuropsiquiatr. 2015. 73: 1019-25
19. Reis CV, Gusmão SN, Elhadi AM, Dru A, Tazinaffo U, Zabramski JM. Midline as a landmark for the position of the superior sagittal sinus on the cranial vault: An anatomical and imaging study. Surg Neurol Int. 2015. 6: 121
20. Samadian M, Nazparvar B, Haddadian K, Rezaei O, Khormaee F. The anatomical relation between the superior sagittal sinus and the sagittal suture with surgical considerations. Clin Neurol Neurosurg. 2011. 113: 89-91
21. Seker A, Martins C, Rhoton AL.editors. Meningeal Anatomy. Meningiomas. Amsterdam: Elsevier; 2010. p. 11-51
22. Shapiro M, Srivatanakul K, Raz E, Litao M, Nossek E, Nelson PK. Dural venous channels: Hidden in plain sight-reassessment of an under-recognized entity. AJNR Am J Neuroradiol. 2020. 41: 1434-40
23. Tubbs RS, Salter G, Elton S, Grabb PA, Oakes WJ. Sagittal suture as an external landmark for the superior sagittal sinus. J Neurosurg. 2001. 94: 985-7