- Gamma Knife Center, Hôpital Erasme, 1070 Brussels, Belgium
- Department of Neurosurgery, Hôpital Erasme, 1070 Brussels, Belgium
- Department of ENT, Hôpital Erasme, 1070 Brussels, Belgium
- Department of Radiophysics, Institut Jules Bordet, 1000 Brussels, Belgium
- Department of Radiation Therapy, Institut Jules Bordet, 1000 Brussels, Belgium
Correspondence Address:
Nicolas Massager
Gamma Knife Center, Hôpital Erasme, 1070 Brussels, Belgium
Department of Neurosurgery, Hôpital Erasme, 1070 Brussels, Belgium
DOI:10.4103/2152-7806.166173
Copyright: © 2015 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Lonneville S, Delbrouck C, Cécile Renier, Devriendt D, Massager N. Repeat Gamma Knife surgery for vestibular schwannomas. Surg Neurol Int 28-Sep-2015;6:153
How to cite this URL: Lonneville S, Delbrouck C, Cécile Renier, Devriendt D, Massager N. Repeat Gamma Knife surgery for vestibular schwannomas. Surg Neurol Int 28-Sep-2015;6:153. Available from: http://surgicalneurologyint.com/surgicalint_articles/repeat-gamma-knife-surgery-for-vestibular-schwannomas/
Abstract
Background:Gamma Knife (GK) surgery is a recognized treatment option for the management of small to medium-sized vestibular schwannoma (VS) associated with high-tumor control and low morbidity. When a radiosurgical treatment fails to stop tumor growth, repeat GK surgery can be proposed in selected cases.
Methods:A series of 27 GK retreatments was performed in 25 patients with VS; 2 patients underwent three procedures. The median time interval between GK treatments was 45 months. The median margin dose used for the first, second, and third GK treatments was 12 Gy, 12 Gy, and 14 Gy, respectively. Six patients (4 patients for the second irradiation and 2 patients for the third irradiation) with partial tumor regrowth were treated only on the growing part of the tumor using a median margin dose of 13 Gy. The median tumor volume was 0.9, 2.3, and 0.7 cc for the first, second, and third treatments, respectively. Stereotactic positron emission tomography (PET) guidance was used for dose planning in 6 cases.
Results:Mean follow-up duration was 46 months (range 24–110). At the last follow-up, 85% of schwannomas were controlled. The tumor volume decreased, remained unchanged, or increased after retreatment in 15, 8, and 4 cases, respectively. Four patients had PET during follow-up, and all showed a significant metabolic decrease of the tumor. Hearing was not preserved after retreatment in any patients. New facial or trigeminal palsy did not occur after retreatment.
Conclusions:Our results support the long-term efficacy and low morbidity of repeat GK treatment for selected patients with tumor growth after initial treatment.
Keywords: Acoustic neuroma, Gamma Knife, irradiation, radiosurgery, recurrence, regrowth, retreatment, vestibular schwannoma
INTRODUCTION
Gamma Knife (GK) surgery has become a recognized treatment option for the management of small to medium-sized vestibular schwannoma (VS). The treatment aims to achieve long-term tumor control and to preserve the function of cranial nerves. Using low prescription doses, GK treatment offers high-tumor control with low morbidity.[
MATERIALS AND METHODS
The present study was approved by the local institutional review board of the Ethical Committee of ULB - Hôpital Erasme (ref. P2015/207). Informed consent was obtained.
Indication for repeat Gamma Knife surgery
We performed a retrospective analysis of the clinical and radiological data for a series of patients treated radiosurgically more than once for the same VS in our department. After the first GK procedure, all these patients were followed up regularly by serial magnetic resonance imaging (MRI) and clinical evaluations in our clinic. A transient tumor volume increase after irradiation occurred occasionally and was not considered as tumor growth from failed GK surgery. Treatment failure was defined as a significant growth of a part or the entire tumor volume after a minimum period of 18 months with an increased growth rate after this period, possibly associated with worsening of cranial nerve function. In fact that the tumor growth rate increased after the usual period of transient volume expansion following GK irradiation was a major criterion to determine the failure of previous treatment. Repeat GK surgery was performed in patients with the following criteria: Previous GK treatment failure as defined above, no significant brainstem deviation due to excessive tumor size, no cystic component into the tumor, and patient preference to undergo new GK rather than microsurgery after full discussion of both treatment options. Three patients had microsurgical removal of a part of the tumor before GK retreatment.
Radiosurgery technique
Detailed information on the benefits and risks of GK retreatment was provided and discussed specifically for all patients according to their particular medical situation. All patients gave informed consent for new GK treatment. Preoperative evaluations, treatment procedures, and follow-up examinations were performed in accordance with the ethical standards of the Belgian Committee on human experimentation and with the Helsinki Declaration.
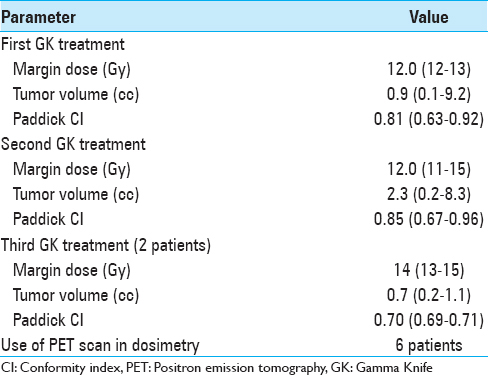
All patients underwent a radiosurgical procedure with the Leksell GK C (from July 2004 to September 2005), 4C (from September 2004 to June 2010), or Perfexion (from July 2010). The Leksell G stereotactic frame was applied to the patient's head under local anesthesia with mild intravenous sedation. For all patients, we performed MRI to acquire stereotactic axial three-dimensional (3D) gadolinium-enhanced T1-weighted and T2-weighted sequences, followed by a computed tomography (CT) densitometric scan. For retreatment, 6 patients also underwent a stereotactic positron emission tomography (PET) acquisition with methionine as the radiotracer, providing a set of 63 planes with a slice thickness of 2.4 mm for dose planning.[
Patient follow-up
Follow-up evaluations were scheduled every 6 months for the 1st year, and annually thereafter if the patient's condition was stable, and tumor volume remained unchanged or reduced on MRI. When the tumor increased, MRI was performed at 6-month intervals. Evolution of the tumor volume was assessed on MRIs co-registered on the follow-up module of Leksell GammaPlan 9.0. This module allows fusion of co-registered images in 3D and projection of the contours of the target volume and prescription isodose in follow-up images. Several volume measurements, as well as accurate evaluation of local or global tumor growth, can be performed with this advanced software. Tumor response was classified into three categories according to tumor volume as follows: Decreased volume (more than 10% volume reduction as compared to the volume at the last GK treatment), stable volume (volume variation within 10% of the volume at the last GK treatment), and increased volume (more than 10% volume enlargement as compared to the volume at the last GK treatment). Tumor metabolic response on PET-methionine was assessed by variations in a semi-quantitative metabolic scale from 0 to 3:0 = hypometabolic, 1 = isometabolic, 2 = moderately hypermetabolic, and 3 = very hypermetabolic, as compared to normal brain parenchyma.
RESULTS
Patient characteristics
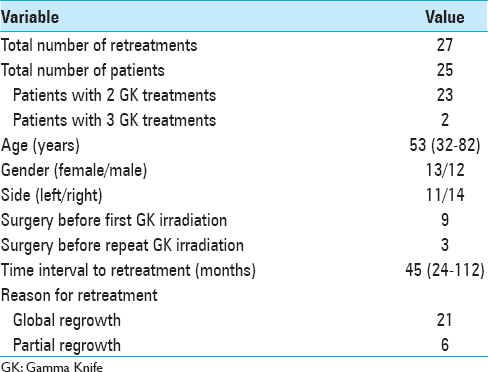
We analyzed data from a series of 27 GK treatments carried out in 25 patients (13 women and 12 men) treated more than once for the same VS in our GK center between 2004 and 2013 [
The median age at the time of retreatment was 53 years (range 32–82). We treated 11 VS located on the left side and 14 VS located on the right side. Neurofibromatosis Type 2 disease was not present. Nine patients (36%) had tumor resection before the first GK irradiation and 3 patients had microsurgery after failure of the first radiosurgery treatment. The median time interval between GK treatments was 45 months (range 24–112). The indication for re-irradiation was global tumor regrowth for 21 retreatments and partial tumor regrowth for six retreatments. Patient hearing status was categorized by the Gardner-Robertson (GR) classification.[
Dosimetric parameters
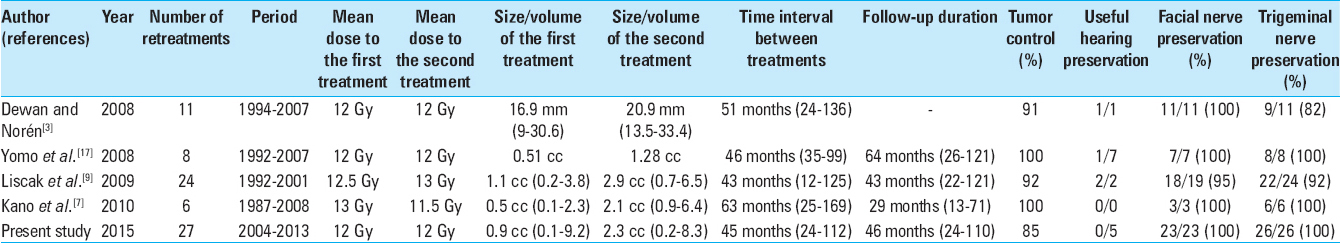
As shown in
Tumor volume response of repeat Gamma Knife treatment
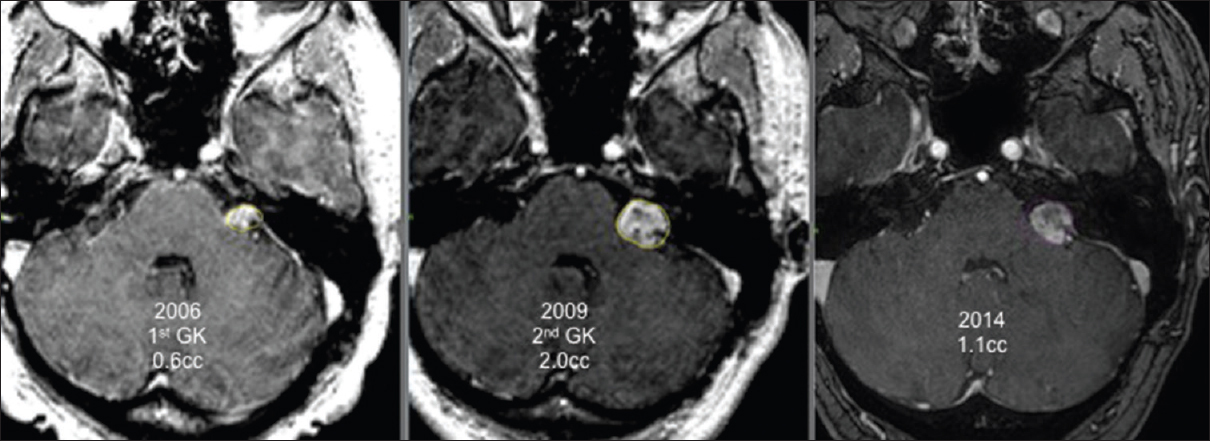
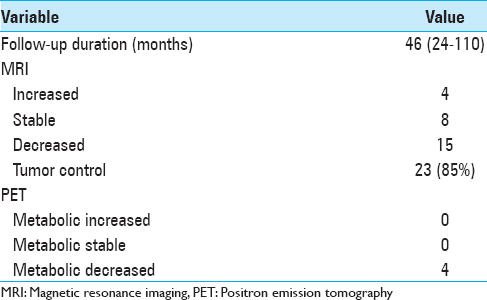
All patients were followed up clinically and radiologically for a minimum of 24 months after the last GK treatment. The mean follow-up duration was 46 months (range 24–110). No patient died during follow-up. At the last follow-up, 15 tumors had decreased in volume [Figures
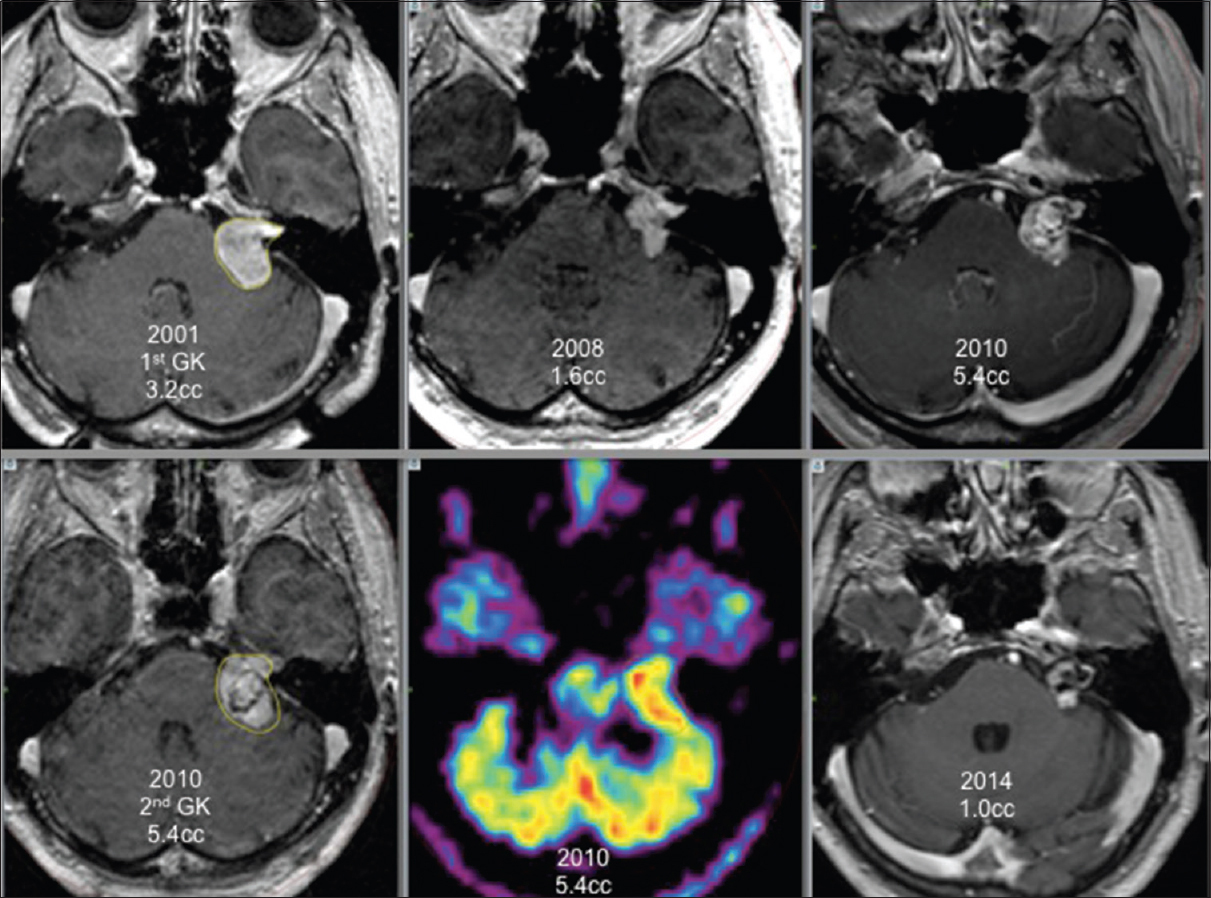
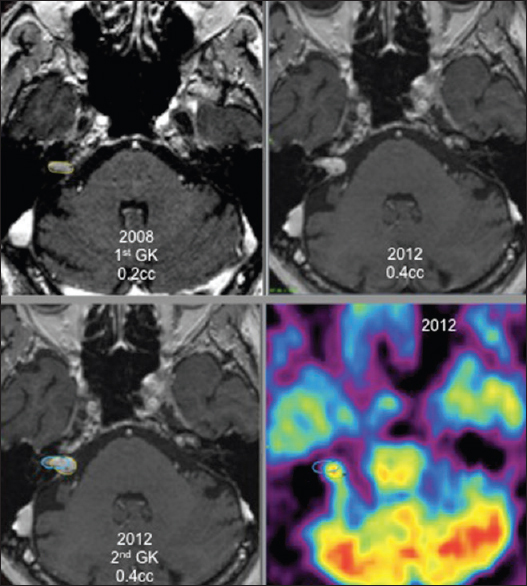
For 6 patients, the new GK treatment focused only on an area inside the tumor that showed local growth as the rest of the tumor did not grow. These patients had a favorable outcome with necrosis at the targeted volume and stopping of tumor growth [
Four of these 6 patients, retreated under stereotactic PET guidance [Figures
Clinical outcome
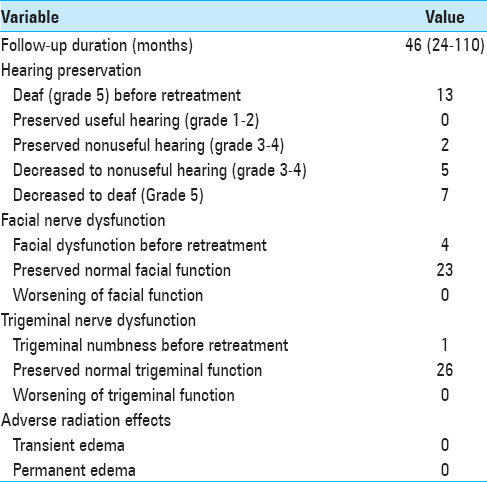
A hearing test performed before the second GK surgery showed that 11 patients were deaf (GR Grade 5), 5 patients preserved useful hearing (GR Grade 1–2), and 9 patients preserved nonuseful hearing (GR Grade 3–4). Both patients treated by three GK procedures were deaf before the third irradiation. After retreatment, all patients were deaf [
Four patients had facial paralysis before retreatment due to the initial microsurgery procedure performed before the first GK treatment. Of the 23 s or third GK irradiation procedures performed on patients with an initially normal nerve function, neither permanent nor transient facial nerve paresis was observed at the outcome. One patient had facial numbness before retreatment that remained unchanged during follow-up. All other patients had preserved normal trigeminal function after the second and third GK procedures. No other neurological deficit related to repeated GK surgery occurred.
DISCUSSION
GK surgery has emerged as an alternative therapeutic option for patients with VS. This treatment has been shown to be a valuable therapy with high, long-term efficacy, and low complication rates.[
Indication for re-irradiation
Transient tumor expansion after GK radiosurgery for VS is a well-known phenomenon that has been reported in 17–74% of patients in different series.[
For patients who require additional treatment after a failed first GK procedure, the selection criteria are very similar to those used for the first GK treatment. Surgical resection must be proposed for patients with large tumors that create symptoms due to a mass effect on the brainstem or other structures such as cerebellar ataxia or motor weakness.[
Few studies have reported the results of a second GK treatment for VS.[
Total versus partial re-irradiation
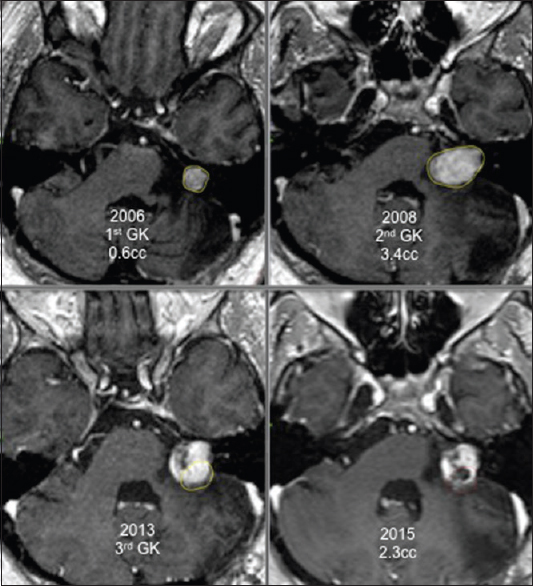
Our series included six GK re-treatments targeting only a part of the tumor. For these patients, thorough analysis of the tridimensional volumetric evolution of the schwannoma after radiosurgery showed clearly that only a part of the tumor was growing while the rest of the tumor volume was controlled by prior irradiation. We performed new GK treatment focused only to the growing area of the tumor to reduce the risks of the added radiation dose delivered to the cranial nerves and the brainstem. After a median follow-up of 42 months, all patients retreated with a new irradiation focused only on the growing part of the tumor had a significant reduction of the targeted volume and achieved whole tumor control.
Third Gamma Knife irradiation
Two patients were treated thrice with GK surgery. In both cases, the last irradiation was done only to the growing part of the tumor. An example of this state is provided in
Tumor control
In the efforts for facial and hearing preservation following GK surgery, the pioneers of GK treatment for VS have decreased gradually the prescription dose used. For almost 20 years, a reduced margin dose of 12 to 13 Gy is commonly used.[
Our results showed that for selected patients, new GK treatment for VS that continue to grow after a prior GK irradiation could be associated with a high rate of tumor control. We observed that 85% of the tumors retreated stopped growing after treatment and that 55% had a significant tumor volume reduction. To our knowledge, only four authors have reported the results of repeat GK treatment for growing VS [
Functional outcome
In our experience, the results of hearing outcome after repeat GK treatment were poor. Five patients with useful hearing were retreated, but none of them retained serviceable hearing during follow-up. However, half of the noncophotic patients who underwent re-irradiation had a GR 3 hearing level at the last control. Repeat GK surgery seems to accelerate hearing deterioration. The accumulated radiation dose delivered to the cochlea or intracanalicular part of the tumor might have contributed to hearing impairment.[
Functional preservation of the facial nerve represented one of the most critical challenges of GK retreatments. None of the 23 retreatments performed on patients with the prior normal function of the seventh cranial nerve induced any facial paralysis, even partial, or transient. One case of facial worsening after a second GK irradiation of VS was reported by Liscak et al.[
One of our patients had facial numbness before retreatment that remained unchanged during follow-up. All other patients retained intact trigeminal nerve function after GK retreatments. In the literature, the preservation rate of the fifth cranial nerve function ranges from 82% to 100%.[
Adverse radiation effects
None of our patients showed imaging evidence of adverse radiation effects (ARE) after retreatment. Dewan and Norén reported slight peduncular edema in 2 patients after a second GK treatment for VS.[
CONCLUSIONS
When VS regrows after a prior GK procedure and transient volume expansion following irradiation is excluded, GK surgery can be proposed as an alternative to microsurgery when the tumor volume remains within the usual radiosurgical range. Although functional hearing could not be preserved after GK retreatment, none of our patients developed facial or trigeminal nerves dysfunction after a second or third irradiation. This retreatment can serve as an alternative therapy to microsurgical resection in order to improve the preservation of cranial nerve function.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Delbrouck C, Hassid S, Choufani G, De Witte O, Devriendt D, Massager N. Hearing outcome after Gamma Knife radiosurgery for vestibular schwannoma: A prospective Belgian clinical study. B-ENT. 2011. 7: 77-84
2. Delsanti C, Roche PH, Thomassin JM, Régis J. Morphological changes of vestibular schwannomas after radiosurgical treatment: Pitfalls and diagnosis of failure. Prog Neurol Surg. 2008. 21: 93-7
3. Dewan S, Norén G. Retreatment of vestibular schwannomas with Gamma Knife surgery. J Neurosurg. 2008. 109: 144-8
4. Flickinger JC, Kondziolka D, Niranjan A, Maitz A, Voynov G, Lunsford LD. Acoustic neuroma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys. 2004. 60: 225-30
5. Gardner G, Robertson JH. Hearing preservation in unilateral acoustic neuroma surgery. Ann Otol Rhinol Laryngol. 1988. 97: 55-66
6. Hasegawa T, Kida Y, Kato T, Iizuka H, Kuramitsu S, Yamamoto T. Long-term safety and efficacy of stereotactic radiosurgery for vestibular schwannomas: Evaluation of 440 patients more than 10 years after treatment with Gamma Knife surgery. J Neurosurg. 2013. 118: 557-65
7. Kano H, Kondziolka D, Niranjan A, Flannery TJ, Flickinger JC, Lunsford LD. Repeat stereotactic radiosurgery for acoustic neuromas. Int J Radiat Oncol Biol Phys. 2010. 76: 520-7
8. Levivier M, Massager N, Wikler D, Lorenzoni J, Ruiz S, Devriendt D. Use of stereotactic PET images in dosimetry planning of radiosurgery for brain tumors: Clinical experience and proposed classification. J Nucl Med. 2004. 45: 1146-54
9. Liscak R, Vladyka V, Urgosik D, Simonova G, Vymazal J. Repeated treatment of vestibular schwannomas after Gamma Knife radiosurgery. Acta Neurochir (Wien). 2009. 151: 317-24
10. Massager N, De Smedt F, Devriendt D. Long-term tumor control of benign intracranial tumors after Gamma Knife radiosurgery in 280 patients followed more than 5 years. Acta Neurol Belg. 2013. 113: 463-7
11. Massager N, Nissim O, Delbrouck C, Delpierre I, Devriendt D, Desmedt F. Irradiation of cochlear structures during vestibular schwannoma radiosurgery and associated hearing outcome. J Neurosurg. 2007. 107: 733-9
12. Massager N, Nissim O, Delbrouck C, Devriendt D, David P, Desmedt F. Role of intracanalicular volumetric and dosimetric parameters on hearing preservation after vestibular schwannoma radiosurgery. Int J Radiat Oncol Biol Phys. 2006. 64: 1331-40
13. Mindermann T, Schlegel I. How to distinguish tumor growth from transient expansion of vestibular schwannomas following Gamma Knife radiosurgery. Acta Neurochir (Wien). 2014. 156: 1121-3
14. Nagano O, Higuchi Y, Serizawa T, Ono J, Matsuda S, Yamakami I. Transient expansion of vestibular schwannoma following stereotactic radiosurgery. J Neurosurg. 2008. 109: 811-6
15. Nagano O, Serizawa T, Higuchi Y, Matsuda S, Sato M, Yamakami I. Tumor shrinkage of vestibular schwannomas after Gamma Knife surgery: Results after more than 5 years of follow-up. J Neurosurg. 2010. 113: 122-27
16. Paddick I. A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J Neurosurg. 2000. 93: 219-22
17. Pollock BE. Management of vestibular schwannomas that enlarge after stereotactic radiosurgery: Treatment recommendations based on a 15 year experience. Neurosurgery. 2006. 58: 241-8
18. Yomo S, Arkha Y, Delsanti C, Roche PH, Thomassin JM, Régis J. Repeat Gamma Knife surgery for regrowth of vestibular schwannomas. Neurosurgery. 2009. 64: 48-54
19. Yu CP, Cheung JY, Leung S, Ho R. Sequential volume mapping for confirmation of negative growth in vestibular schwannomas treated by Gamma Knife radiosurgery. J Neurosurg. 2000. 93: 82-9






Jesus Farinas Yanes
Posted November 16, 2016, 2:24 pm
Awesone work about radiosurgery and tumor.