- Department of Neurosurgery, Fukuoka Children’s Hospital, Fukuoka, Japan
- Department of Psychiatry, Shourai Hospital, Karatsu, Japan
- Department of Neurosurgery, Kyushu University, Fukuoka, Japan
- Department of Neurosurgery, Iizuka Hospital, Iizuka, Japan
- Department of Neurosurgery, Hachisuga Hospital, Munakata, Japan.
Correspondence Address:
Ai Kurogi, Department of Neurosurgery, Fukuoka Children’s Hospital, Fukuoka, Japan.
DOI:10.25259/SNI_479_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ai Kurogi1, Nobuya Murakami1, Satoshi O. Suzuki2, Takafumi Shimogawa3, Nobutaka Mukae4, Koji Yoshimoto3, Takato Morioka5. Retained medullary cord and caudal lipoma with histopathological presence of terminal myelocystocele in the epidural stalk. 04-Aug-2023;14:279
How to cite this URL: Ai Kurogi1, Nobuya Murakami1, Satoshi O. Suzuki2, Takafumi Shimogawa3, Nobutaka Mukae4, Koji Yoshimoto3, Takato Morioka5. Retained medullary cord and caudal lipoma with histopathological presence of terminal myelocystocele in the epidural stalk. 04-Aug-2023;14:279. Available from: https://surgicalneurologyint.com/surgicalint-articles/12481/
Abstract
Background: The retained medullary cord (RMC), caudal lipoma, and terminal myelocystocele (TMCC) are thought to originate from the failed regression spectrum during the secondary neurulation, and the central histopathological feature is the predominant presence of a central canal-like ependyma-lined lumen (CC-LELL) with surrounding neuroglial tissues (NGT), as a remnant of the medullary cord. However, reports on cases in which RMC, caudal lipoma, and TMCC coexist are very rare.
Case Description: We present two patients with cystic RMC with caudal lipoma and caudal lipoma with an RMC component, respectively, based on their clinical, neuroradiological, intraoperative, and histopathological findings. Although no typical morphological features of TMCC were noted on neuroimaging, histopathological examination revealed that a CC-LELL with NGT was present in the extraspinal stalk, extending from the skin lesion to the intraspinal tethering tract.
Conclusion: This histopathological finding indicates the presence of TMCC that could not be completely regressed and further supports the idea that these pathologies can be considered consequences of a continuum of regression failure during secondary neurulation.
Keywords: Caudal lipoma, Closed spinal dysraphism, Epidural stalk, Retained medullary cord, Secondary neurulation failure, Terminal myelocystocele
INTRODUCTION
Although the precise mechanism of the secondary neurulation remains unknown, it is generally speculated that it begins with mesenchymal epithelium transformation within a pluripotent blastema called the caudal cell mass.[
The retained medullary cord (RMC) is an entity of closed spinal dysraphism characterized by a redundant nonfunctional “cord-like structure (C-LS)” continuous from the functional conus and extending to the dural cul-de-sac, which leads to cord tethering.[
Although diagnostic criteria for RMC have not yet been fully established, the following three items are generally considered important:[
The terminal myelocystocele (TMCC) is an unusual type of closed spinal dysraphism, characterized by cystic dilatation of the terminal portion of the central canal with a trumpet-like configuration that herniates through a spinal bifida.[
Recently, we treated two patients with RMC and caudal lipoma. While no typical morphological features of TMCC were observed on neuroimaging, CC-LELL with NGT was histopathologically recognized in the extraspinal stalk, continuing from the skin lesion to the intraspinal tethering tract, which was suspected to be the coexistence of RMC and TMCC that could not be completely regressed. Here, we report details of clinical, neuroradiological, intraoperative, and histopathological findings of these cases.
CASE REPORT
Patient 1
The boy was detected with an abnormal gluteal cleft at birth and was referred to us at the age of 5 months. A Y-shaped groove continuous from the gluteal cleft was noted [
We performed untethering surgery at the age of 6 months. A skin incision was made to surround the dimple, and a stalk continuous from the subcutaneous fat entered the spinal canal through the myofascial defect [
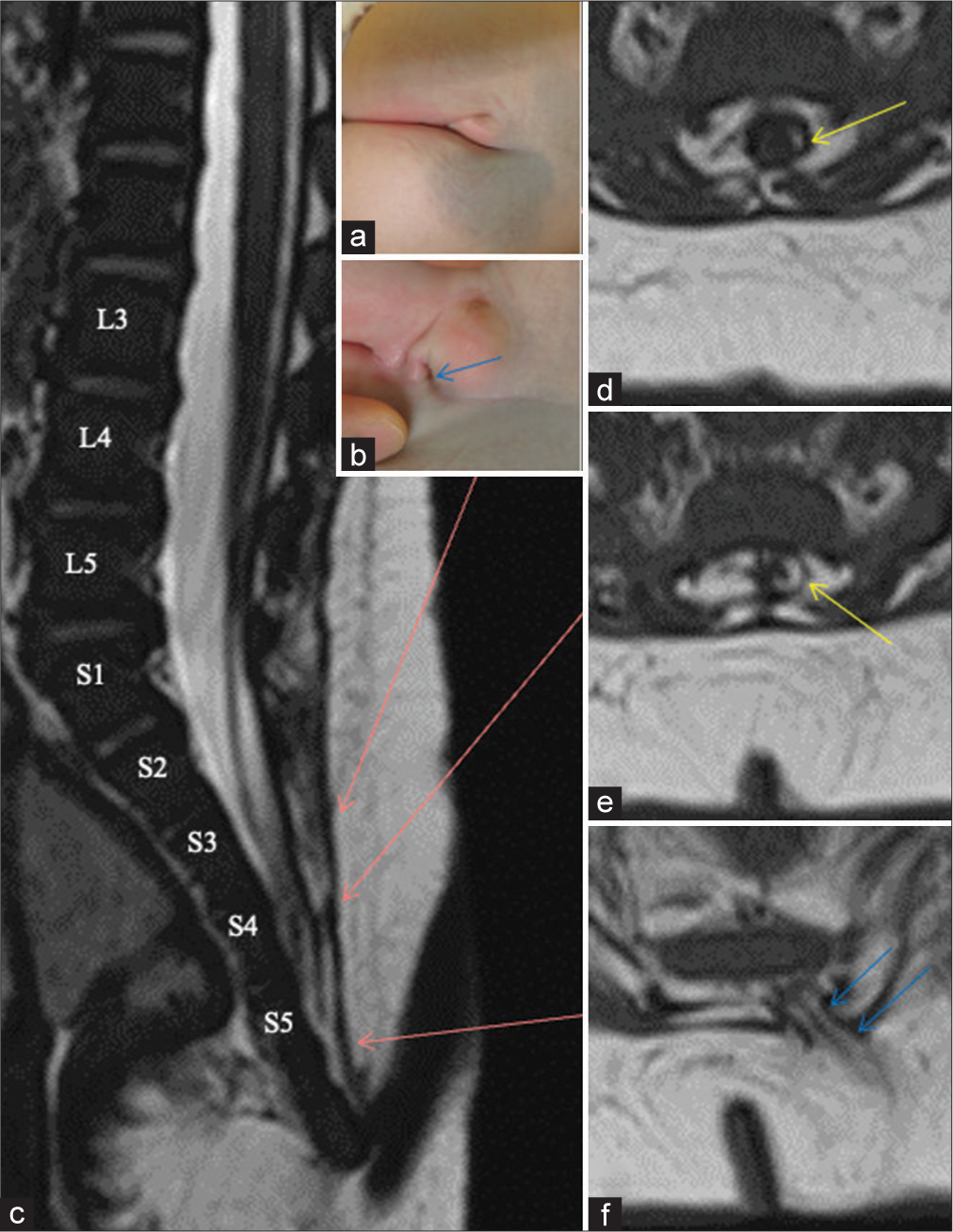
Figure 1:
(Patient 1) (a and b) Photographs of the skin lesion, shows a dimple (blue arrows) under the groove on the right side. (c-f) Sagittal view of 3D-hT2WI (c) and axial views of T1WI (d-f). Each slice level of the axial views (d-f) is indicated by red arrows on (c). Each level of the vertebral columns from L3 to S5 is indicated on (c). The caudal cyst wall is partly composed of lipomatous tissue (d,e, yellow arrows). A stalk extending from the subcutaneous fat into the vertebral canal is shown as blue arrows [panel (f)]. 3D-hT2WI: three-dimensional heavily T2-weighted image, T1WI: T1-weighted image.
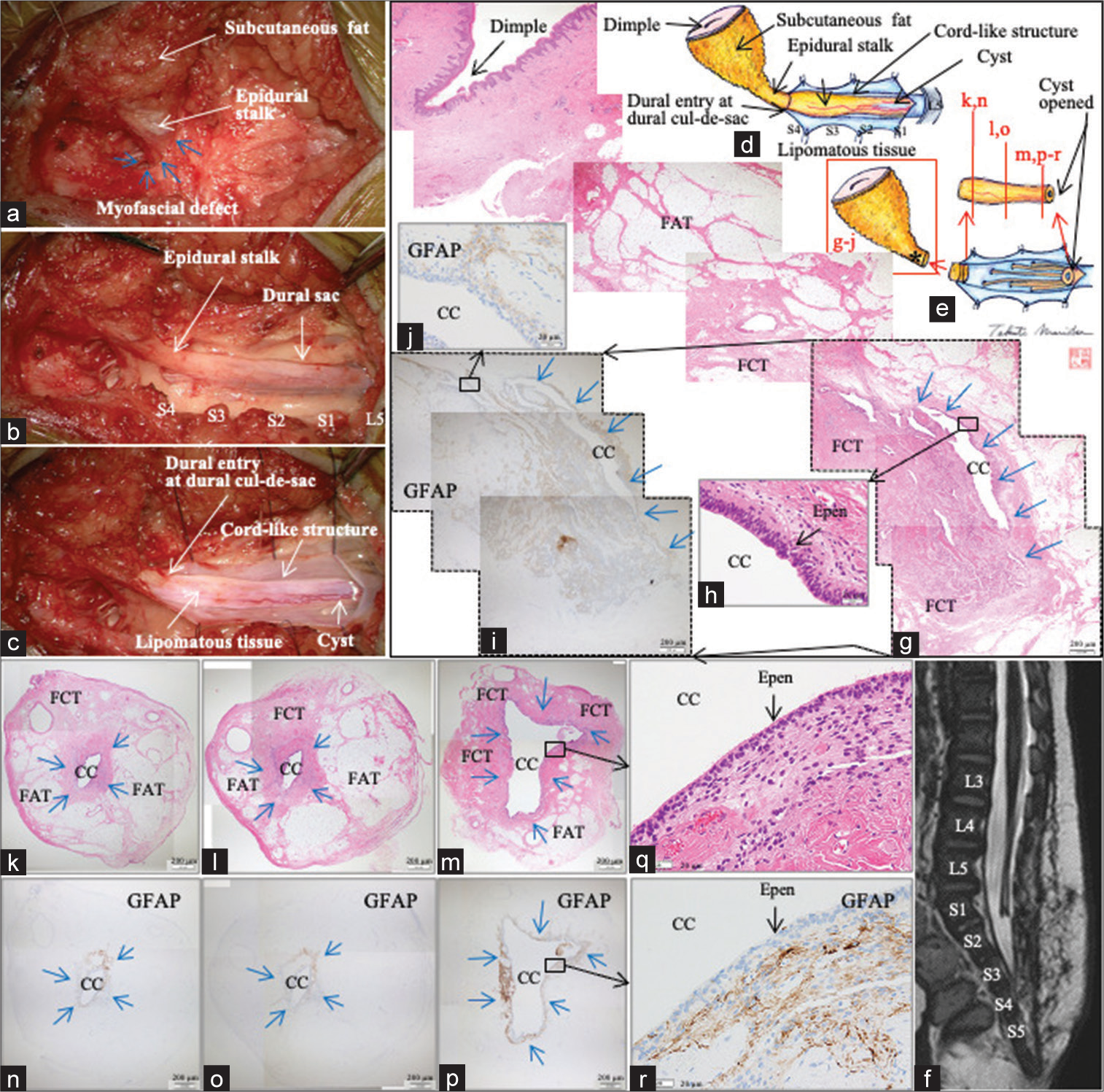
Figure 2:
(Patient 1) (a-c) Microscopic views and (d and e) schematic drawings of the operative findings. Each level of the exposed vertebral arches from L5 to S4 is indicated on (b). (f) Sagittal view of postoperative 3D-hT2WI. Each level of the vertebral columns from L3 to S5 is indicated on (f). (g-i) Photomicrographic composite of the vertical view of the skin and epidural stalk, stained with H&E (g and h) and immunostained for GFAP (i and j). (k-r) Photomicrograph of the axial sections of the cord-like structure stained with H&E (k-m and q) and immunostained for GFAP (n-p and r). CC-LELL with NGT is surrounded by blue arrows on (g), (i), and (k-p). The location of each section is indicated as “gr” in (e). The location of each section is indicated in (e). The location of central canal-like ependyma-lined lumen surrounded by neuroglial tissue in the epidural stalk is indicated as * in (e) and (g-j and k-r). The sites surrounded by dotted line in (g and i) indicate the same site. Higher magnification views of the areas indicated by the square in (g, i, m, and p) are shown in (h, j, q, and r), respectively. 3D-hT2WI, three dimensional heavily T2-weighted image, H&E: hematoxylin and eosin, CC-LELL, central canal-like ependyma-lined lumen; GFAP: glial fibrillary acidic protein, FAT: fibroadipose tissue, FCT: fibrocollagenous tissue, CC: central canal-like structure, and Epen: ependymal cells.
After confirming that the exposed C-LS in the operative field was nonfunctional with intraoperative neurophysiological monitoring (IONM) as previously described,[
Postoperatively, no de novo neurological abnormalities were observed. MRI performed on the 7th postoperative day demonstrated successful untethering of the cord [
Histopathologically, the distal side of the epidural stalk, continuing from the skin dimple, was mainly composed of FAT, while the proximal side was mainly composed of FCT [
Patient 2
The boy, with joint contractures in the four limbs due to arthrogryposis multiplex congenita, had an abnormal gluteal cleft with skin lesions at birth [
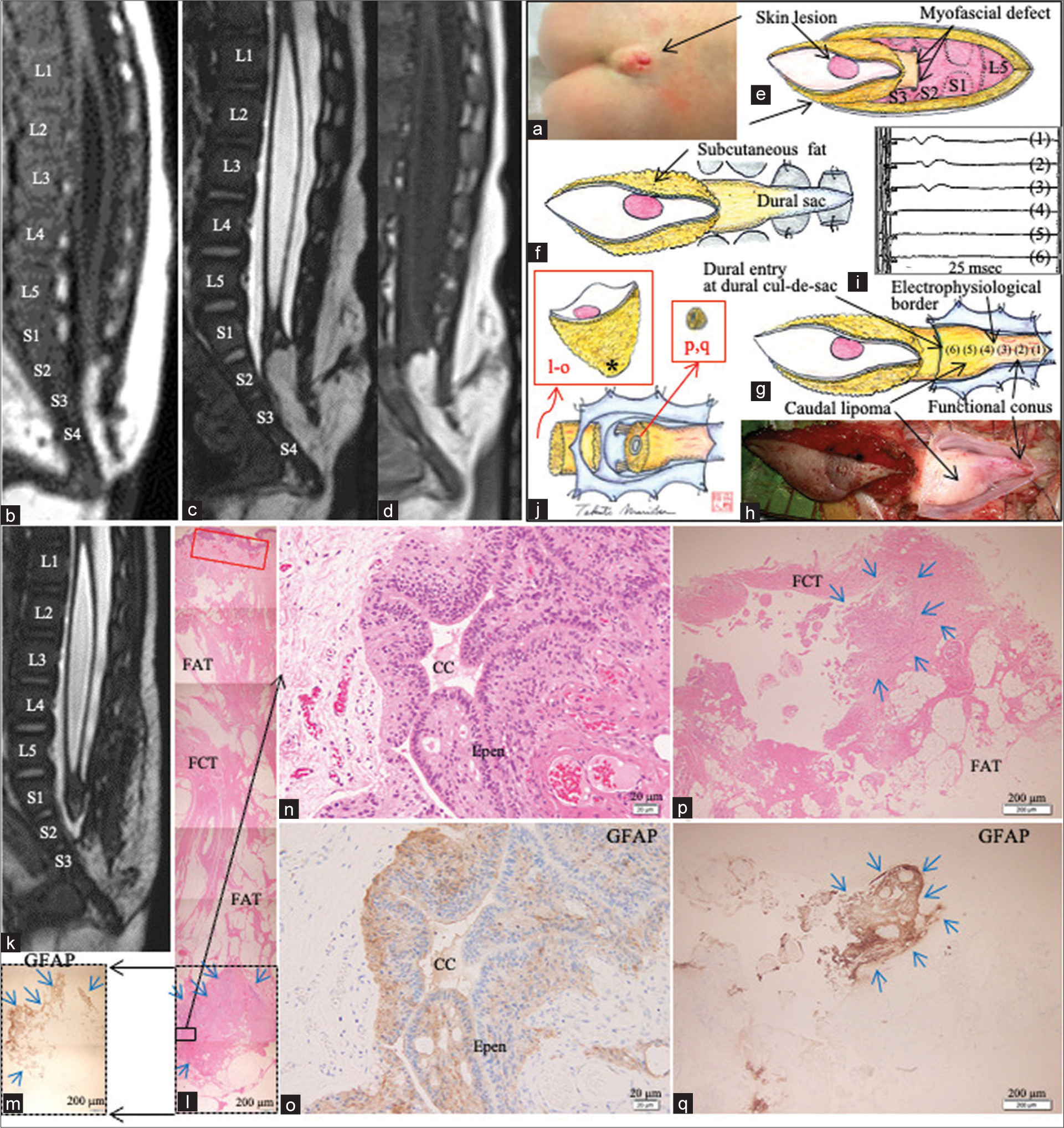
Figure 3:
(Patient 2) (a) Photograph of the skin lesion. (b) Sagittal view of T1WI at the age of 2 months, (c and d) sagittal views of 3D-hT2WI (c) and T1WI (d) at the age of 7 months. Each level of the vertebral columns from L3 to S5 is indicated on (b) and (c). and (e-i) Schematic drawings (e-g and i) and microscopic view (h) of the operative findings. (j) The electrophysiological border between functional conus and caudal lipoma was determined with IONM, by tracing the evoked compound muscle potentials of the external anal sphincter with stimulation of 2 mA, beginning from the functional conus (g-(1)(2)(3)), and proceeding to the caudal lipoma (g-(4)(5)(6)). Stimulation sites are indicated on (g). Each histopathology section is shown in red characters “l-o, p/q”. (k) Sagittal view of postoperative 3D-hT2WI. (l-o) Photomicrographic composite of the vertical view of the skin and epidural stalk, stained with H&E (l and n) and immunostained for GFAP (m and o). CC-LELL with NGT is surrounded by blue arrows on (m), (l), (p), and (q). The location of CC-LELL with NGT surrounded by neuroglial tissue in the epidural stalk is indicated as * in (j). The sites surrounded by dotted line in (l and m) indicate the same site. Higher magnification views of the area indicated by the square in (l) are shown in (n and o), respectively. (p and q) Photomicrograph of the cyst wall in the lipoma stained with H&E (p) and immunostained for GFAP (q). The location of each section is indicated as l-o and p, q in (j). T1WI: T1-weighted image, 3D-hT2WI: three-dimensional heavily T2-weighted image, IONM: Intraoperative neurophysiological monitoring, H&E: hematoxylin and eosin, CC-LELL, central canal-like ependyma-lined lumen; GFAP: glial fibrillary acidic protein, FAT: fibroadipose tissue, FCT: fibrocollagenous tissue, cc: central canal-like structure, and Epen: ependymal cells.
Neurologically, there was little movement of the four limbs, and in particular, no movement of the bilateral ankle joints was observed. However, regular urination and defecation were observed. The second MRI revealed that the caudal lipoma increased in size and extension to the subcutaneous fat through an enlarged sacral hiatus became apparent [
We performed untethering surgery at 7 months. A skin incision was made to surround the skin lesion, and a fatty stalk continuous from the subcutaneous fat entered the spinal canal through the myofascial defect [
Postoperatively, no de novo neurological abnormalities were observed. MRI performed on the 7th postoperative day demonstrated successful untethering of the cord [
Histopathologically, the skin lesion contained clusters of blood vessels beneath the squamous epithelium [
DISCUSSION
The C-LS of Patient 1 satisfied items (1) and (3) of the three items for diagnosing RMC.[
In Patient 2, the caudal lipoma was tethered to the spinal cord, and the electrophysiological border was identified at the morphological border between the two. There was a tiny cyst within the lipoma, and there was GFAP-positive NGT in its wall. Since the CC-LELL was not entirely included in the specimen,[
The most prominent histopathological finding of the epidural stalk in the present cases was that the CC-LELL surrounded by NGT, which is also a characteristic finding of TMCC, was observed in the FCT, while no typical morphological features of TMCC were noted on neuroimaging. Direct communication between the CC-LELL and RMC has not been histopathologically proven. However, because the CC-LELL was located on the proximal side of the epidural stalk, it is possible that there was an originally communication between them. Therefore, this histopathological finding indicates the presence of TMCC that could not be completely regressed and supports the idea raised by previous authors that TMCC and RMC can be considered consequences of a continuum of regression failure during secondary neurulation.[
However, reports of cases in which RMC and TMCC coexist are extremely rare. To date, only three cases have been reported. Kim et al.[
The most likely reason why the histopathological coexistence of TMCC and RMC tissues has rarely been reported, as in these cases, is that the tethering tract caused by secondary neurulation failures, such as RMC and caudal lipoma, is rarely continuous from the skin lesion to the spinal canal through the myofascial defect and spina bifida,[
Untethering of the cord was the best surgical method for both patients. Another important issue is the surgical strategy used to treat cystic lesions. In Patient 1, the cyst was present in the nonfunctional RMC; therefore, untethering was performed at the site of the cystic RMC, which directly opened the cyst, leading to postoperative reduction in its size as previously reported.[
Furthermore, an active histopathological search of the epidural stalk revealed a small TMCC tissue. Future studies are expected to increase the number of cases with coexistence of TMCC and RMC.
CONCLUSION
This histopathological finding indicates the presence of TMCC that could not be completely regressed and further supports the idea that these three pathologies can be consequences of a continuum of regression failure during secondary neurulation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Research Foundation of Fukuoka Children’s Hospital.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Hashiguchi K, Morioka T, Samura K, Yoshida F, Miyagi Y, Nagata S. Holocord hydrosyringomyelia with terminal myelocystocele revealed by constructive interference in steady-state MR imaging. Pediatr Neurosurg. 2008. 44: 509-12
2. Kim KH, Lee JY, Wang KC. Secondary neurulation defects-1: Retained medullary cord. J Korean Neurosurg Soc. 2020. 63: 314-20
3. Kim KH, Lee JY, Yang J, Park SH, Kim SK, Wang KC. Cystic retained medullary cord in an intraspinal J-shaped cul-de-sac: A lesion in the spectrum of regression failure during secondary neurulation. Childs Nerv Syst. 2021. 37: 2051-6
4. Kurogi A, Murakami N, Morioka T, Mukae N, Shimogawa T, Kudo K. Two cases of retained medullary cord running parallel to a terminal lipoma. Surg Neurol Int. 2021. 12: 112
5. Kurogi A, Murakami N, Mukae N, Shimogawa T, Shono T, Suzuki SO. Retained medullary cord associated with terminal myelocystocele and intramedullary arachnoid cyst. Pediatr Neurosurg. 2022. 57: 184-90
6. Lee JY, Kim SP, Kim SW, Park SH, Choi JW, Phi JH. Pathoembryogenesis of terminal myelocystocele: Terminal balloon in secondary neurulation of the chick embryo. Childs Nerv Syst. 2013. 29: 1683-8
7. McLone DG, Naidich TP. Terminal myelocystocele. Neurosurgery. 1985. 16: 36-43
8. Morioka T, Hashiguchi K, Yoshida F, Matsumoto K, Miyagi Y, Nagata S. Neurosurgical management of occult spinal dysraphism associated with OEIS complex. Childs Nerv Syst. 2008. 24: 723-9
9. Morioka T, Murakami N, Ichiyama M, Kusuda T, Suzuki SO. Congenital dermal sinus elements in each tethering stalk of coexisting thoracic limited dorsal myeloschisis and retained medullary cord. Pediatr Neurosurg. 2020. 55: 380-7
10. Morioka T, Murakami N, Kanata A, Tsukamoto E, Suzuki SO. Retained medullary cord with sacral subcutaneous meningocele and congenital dermal sinus. Childs Nerv Syst. 2020. 36: 423-7
11. Morioka T, Murakami N, Kurogi A, Mukae N, Shimogawa T, Shono T. Embryopathological relationship between retained medullary cord and caudal spinal lipoma. Interdiscip Neurosurg. 2022. 29: 101534
12. Morioka T, Murakami N, Suzuki SO, Mukae N, Shimogawa T, Kurogi A. Surgical histopathology of a filar anomaly as an additional tethering element associated with closed spinal dysraphism of primary neurulation failure. Surg Neurol Int. 2021. 12: 373
13. Morota N, Ihara S, Ogiwara H. New classification of spinal lipomas based on embryonic stage. J Neurosurg Pediatr. 2017. 19: 428-39
14. Mukae N, Morioka T, Suzuki SO, Murakami N, Shimogawa T, Kanata A. Two cases of large filar cyst associated with terminal lipoma: Relationship with retained medullary cord. World Neurosurg. 2020. 142: 294-8
15. Murakami N, Morioka T, Shimogawa T, Hashiguchi K, Mukae N, Uchihashi K. Retained medullary cord extending to a sacral subcutaneous meningocele. Childs Nerv Syst. 2018. 34: 527-33
16. Murakami N, Morioka T, Shimogawa T, Mukae N, Inoha S, Sasaguri T. Ependyma-lined canal with surrounding neuroglial tissues in lumbosacral lipomatous malformations: Relationship with retained medullary cord. Pediatr Neurosurg. 2018. 53: 387-94
17. Pang D, Chong S, Wang KC, Di Rocco C, Pang D, Rutka J, editors. Secondary neurulation defects-1: Thickened filum terminale, retained medullary cord. Textbook of Pediatric Neurosurgery. Switzerland: Springer; 2020a. p.
18. Pang D, Lee JY, Wang KC, Di Rocco C, Pang D, Rutka JT, editors. Secondary neurulation defects-2: Terminal myelocystocele: Surgical observations, laboratory findings, and theory of embryogenesis. Textbook of Pediatric Neurosurgery. Switzerland: Springer; 2020b. p.
19. Pang D, Zovickian J, Lee JY, Moes GS, Wang KC. Terminal myelocystocele: Surgical observations and theory of embryogenesis. Neurosurgery. 2012. 70: 1383-405 discussion 1404-5
20. Pang D, Zovickian J, Moes GS. Retained medullary cord in humans: Late arrest of secondary neurulation. Neurosurgery. 2011. 68: 1500-19 discussion 1519
21. Sala F, Barone G, Tramontano V, Gallo P, Ghimenton C. Retained medullary cord confirmed by intraoperative neurophysiological mapping. Childs Nerv Syst. 2014. 30: 1287-91
22. Shim Y, Park HJ, Kim KH, Park SH, Wang KC, Lee JY. Retained medullary cord and terminal myelocystocele as a spectrum: Case report. Childs Nerv Syst. 2022. 38: 1223-8
23. Shirozu N, Morioka T, Inoha S, Imamoto N, Sasaguri T. Enlargement of sacral subcutaneous meningocele associated with retained medullary cord. Childs Nerv Syst. 2018. 34: 1785-90
24. Yang J, Lee JY, Kim KH, Wang KC. Disorders of secondary neurulation: Mainly focused on pathoembryogenesis. J Korean Neurosurg Soc. 2021. 64: 386-405