- Department of Neurosurgery, Universitas Airlangga - Faculty of Medicine, Dr. Soetomo Academic General Hospital, Surabaya, East Java, Indonesia
- Department of Public Health, Universitas Airlangga - Faculty of Medicine, Dr. Soetomo Academic General Hospital, Surabaya, East Java, Indonesia
Correspondence Address:
Wihasto Suryaningtyas, Department of Neurosurgery, Universitas Airlangga - Faculty of Medicine, Dr. Soetomo Academic General Hospital, Surabaya, East Java, Indonesia.
DOI:10.25259/SNI_848_2024
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Roidah Taqiyya Zahra Wathoni1, Wihasto Suryaningtyas1, Budi Utomo2, Muhammad Arifin Parenrengi1, Agus Turchan1, Asra Al Fauzi1. Risk factors for cerebrospinal fluid shunt infection in pediatrics: A meta-analysis. 24-Jan-2025;16:16
How to cite this URL: Roidah Taqiyya Zahra Wathoni1, Wihasto Suryaningtyas1, Budi Utomo2, Muhammad Arifin Parenrengi1, Agus Turchan1, Asra Al Fauzi1. Risk factors for cerebrospinal fluid shunt infection in pediatrics: A meta-analysis. 24-Jan-2025;16:16. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13351
Abstract
Background: Placement of cerebrospinal fluid (CSF) shunt for diversion remains a primary treatment for patients with hydrocephalus despite its surgical complications, including shunt infection, that remain high and become a medical and social problem. The meta-analysis was conducted to investigate risk factors of shunt infection in pediatrics.
Methods: Literature was searched on PubMed, Scopus, and the Cochrane Library. The methodology used for this investigation was preferred reporting items for systematic reviews and meta-analysis.
Results: This meta-analysis included five publications. The only significant results were found in ages P
Conclusion: Younger age during the shunt placement procedure, Caucasian race, and African–American race have a significantly higher risk of CSF shunt infection. The previously reported higher risk of shunt infection in cohort studies, such as IVH of prematurity and the presence of gastrostomy, were not significant in this study. Primary studies regarding shunt infection are advocated to be performed in a more extensive population with further risk factors included in the analysis.
Keywords: Hydrocephalus, Pediatrics, Risk factors, Shunt infection
INTRODUCTION
Complications are frequent following cerebrospinal fluid (CSF) shunt placement. Infection is one of the most frequent and serious side effects, occurring in 5–25% of cases.[
Several studies with observational methods have previously been carried out to identify risk factors in the occurrence of shunt infections. Despite its various reports, there has been no systematic review and meta-analysis methods ever conducted as the basis for the strongest scientific evidence regarding shunt infection in pediatrics. The authors aim to collect studies that reported risk factors of shunt infection and analyze using a combined proportion system to assess the factors that provide the most impactful and significant role in shunt infection. The results of this research would be beneficial in predicting the incidence of shunt infections more accurately and developing prevention strategies.
MATERIALS AND METHODS
Literature search strategy
The journal search strategy was carried out according to the preferred reporting items for systematic review and meta-analysis protocol (PRISMA) guidelines [
Study selection criteria
The authors reviewed published studies in the database. We excluded all unpublished studies or articles-in-press and used the keywords [
Analysis
RevMan version 5.4 from the Cochrane review was used. A forest plot was generated to show visual statistics between variables. Pooled effect sizes and matching 95% confidence intervals (CIs) were computed for relevant outcome measurements. Cochran’s Q test and the I2 statistic were used to evaluate the heterogeneity among the studies. When necessary, subgroup and sensitivity analyses were carried out to investigate the causes of heterogeneity and evaluate the reliability of the results.
Quality assessment
A bias analysis (confounding, selection, information, and reporting bias) was conducted on five included studies. Authors assessed the bias based on the modified Cochrane collaboration tool: Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) [
Protocol registration
The protocol for this systematic review and meta-analysis was filed with the relevant registry International prospective register of systematic reviews (PROSPERO) to guarantee transparency and adherence to standards. PROSPERO has already received the study’s registration (ID: 598408). In
RESULTS
Characteristics of included studies
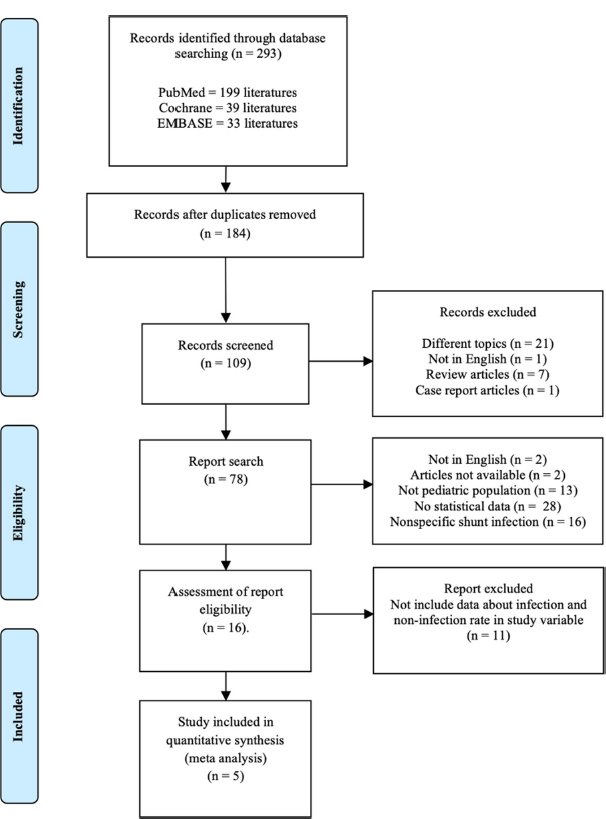
Five studies were included after conducting a literature search. The research flow diagram is shown in
Risk of bias assessment
As shown in
Meta-analysis of measured outcomes
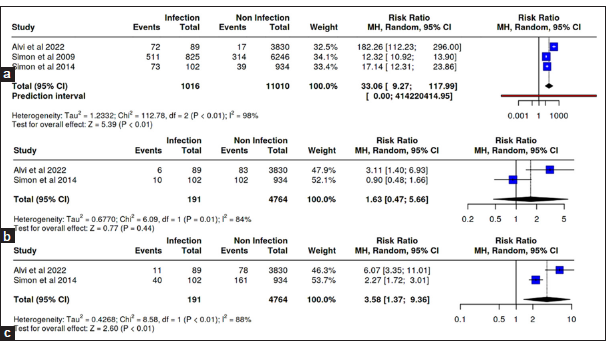
A total of three studies in the <6 months group, two studies in the 6–12 months group, and two studies in the more than 12 months group were reported. The analysis showed a significant increase in the risk of infection in the age group <6 months with RR 33.06 (95% CI 9.27–117.99; P < 0.01) and at age more than 12 months at the time of CSF shunt installation with RR 3.58 (95% CI 1.37-9.36; P < 0.01 [
Four of the five meta-analysis studies reported the incidence of infection after CSF shunt installation in the hydrocephalus group with intraventricular hemorrhage (IVH) of prematurity etiology, three studies in the congenital hydrocephalus etiology group, and four studies in the central nervous system (CNS) tumor etiology group. The relative risk meta-analysis test showed no significant results in all groups [
Two studies in this meta-analysis were analyzed for the incidence of infection after CSF shunt installation in populations with comorbid malignancies. The result demonstrated a significant RR of CSF shunt infection with respiratory comorbidity (RR = 0.22; 95% CI 0.11–0.43; P < 0.01) [
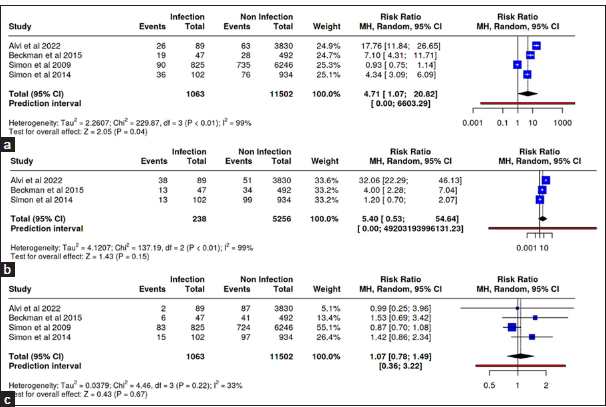
Three studies in this meta-analysis analyzed the incidence of infection after cerebrospinal fluid (CSF) shunt installation in population groups of Caucasian race, three studies in population groups of African–American race, and two studies in population groups with Latin ethnicity. There were 500 incidents of infection from a total of 4775 hydrocephalus groups with Caucasians compared to 484 incidents of infection from a total of 3871 hydrocephalus populations other than Caucasians. The relative risk results demonstrated a significant risk in the probability of CSF shunt infection in the Caucasian hydrocephalus group with an RR of 15.24 (95% CI 6.77–34.34; P < 0.01) and African–American hydrocephalus group with an RR of 2.37 which was statistically significant (95% CI 2.07–2.7; P < 0.01) [
DISCUSSION
Age
This study obtained significant results in the age group of <6 months with an RR of 33.06 (95% CI 9.27–117.99; P < 0.01). This finding is consistent with earlier retrospective reports. There are several hypotheses to expound the relation of age and risk of CSF shunt infection. One of the established theories is an underdevelopment of the cellular and humoral immune systems in children under 12 months of age, where immunoglobulins in newborns are dominated by immunoglobulin G (IgG) obtained from maternal through the placenta. Maternal IgG concentration will decrease progressively due to physiological catabolism processes until 12 months of age. Maternal IgG has limited capacity because it relies on maternal history of antigen exposure. Antibody production begins in the 3rd month of life, and at the age of 4–6 years, the IgG levels will be equivalent to adult IgG levels.[
Etiology of hydrocephalus
IVH of prematurity as the cause of hydrocephalus showed a major significance in this study. Newborns with very low birth weight who survive the critical phase will have a high risk of IVH, including germinal matrix hemorrhage.[
CNS tumors, as the etiology of hydrocephalus, have been identified as a significant risk factor for CSF shunt infections. Hydrocephalus associated with malignancies is frequently observed in pediatric patients with primary brain tumors. Studies indicate that hydrocephalus is present in approximately 55–60% of cases, including 50% where it is diagnosed concurrently with the tumor itself.[
Presence of gastrostomy status
Placement of a CSF shunt is associated with a 2.49-fold increase in the risk of infection in patients undergoing gastrostomy. This increased risk is attributed to an incomplete skin barrier, which facilitates pathogen colonization around the CSF shunt.[
Comorbid diseases
A meta-analysis of comorbid conditions associated with CSF shunt placement revealed heterogeneous findings. However, hydrocephalus with comorbid respiratory diseases significantly increases the risk of CSF shunt infection. Other comorbidities, including respiratory, cardiological, congenital, gastrointestinal, hematological, metabolic, and renal conditions, may elevate infection risk due to prolonged hospitalization, repeated surgeries, or immune deficiency.[
Race and ethnicity
The meta-analysis included participants of Caucasian, African–American, and Latino ethnicities. Significant associations with CSF shunt infections were observed in Caucasians and African–Americans, while no significant link was found in Latinos. The reasons for the higher risk in Caucasians and lower risk in African–Americans remain unclear, and no literature currently explains this disparity. The predominance of Caucasian participants (55.2%) may have influenced the results.
Limitation
The authors did not review ongoing randomized trials because no literature or research reports analyzed risk factors for CSF shunt infection until this study was conducted. The amount of literature that meets the eligibility screening in this meta-analysis is limited, and the population of studies that meet the eligibility screening only comes from the United States, so it does not reflect the world’s global population.
CONCLUSION
Several risk factors, such as younger age during the shunt placement procedure, IVH of prematurity, presence of gastrostomy, African–American race, and respiratory comorbidities, have a significant tendency in the incidence of CSF shunt infection in pediatrics. This meta-analysis provides a foundation for future research with higher levels of evidence, as prior studies primarily relied on cohort designs. Future investigations involving larger, multicenter populations across diverse countries are recommended to achieve a more comprehensive global understanding of these risk factors.
Ethical approval:
Institutional Review Board approval is not required as it is a meta -analysis study.
Declaration of patient consent:
Patient’s consent is not required as there are no patients in this study.
Financial support and sponsorship:
Nil.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Alvi MA, Bhandarkar AR, Daniels DJ, Miller KJ, Ahn ES. Factors associated with early shunt revision within 30 days: Analyses from the National Surgical Quality Improvement Program. J Neurosurg Pediatr. 2022. 29: 21-30
2. Beckman JM, Amankwah EK, Tetreault LL, Tuite GF. Reduction in CSF shunt infection over a 10-year period associated with the application of concentrated topical antibiotic powder directly to surgical wounds prior to closure. J Neurosurg Pediatr. 2015. 16: 648-61
3. Capasso L, Borrelli A, Cerullo J, Pisanti R, Figliuolo C, Izzo F. Role of immunoglobulins in neonatal sepsis. Transl Med UniSa. 2015. 11: 28-33
4. Champeaux-Depond C, Ramasy Razafindratovo RM, Chevret S. Gastrostomy and internal cerebrospinal fluid shunt in adults. A systematic review and meta-analysis of the risk of infection. Neurochirurgie. 2022. 68: e75-83
5. El Rahal A, Cipriani D, Fung C, Hohenhaus M, Sveikata L, Straehle J. Hydrocephalus shunting in supratentorial glioblastoma: functional outcomes and management. Front Oncol. 2022. 12: 796105
6. Gassas A, Kennedy J, Green G, Connolly B, Cohen J, DagEllams U. Risk of ventriculoperitoneal shunt infections due to gastrostomy feeding tube insertion in pediatric patients with brain tumors. Pediatr Neurosurg. 2006. 42: 95-9
7. Grant JP. Percutaneous endoscopic gastrostomy. Ann Surg. 1993. 217: 168-74
8. Justiz-Vaillant AA, Hoyte T, Davis N, Deonarinesingh C, De Silva A, Dhanpaul D. A systematic review of the clinical diagnosis of transient hypogammaglobulinemia of infancy. Children. 2023. 10: 1358
9. Kalia YN, Nonato LB, Lund CH, Guy RH. Development of skin barrier function in premature infants. J Invest Dermatol. 1998. 111: 320-6
10. Kestle JR, Riva-Cambrin J, Wellons JC, Kulkarni AV, Whitehead WE, Walker ML. A standardized protocol to reduce cerebrospinal fluid shunt infection: The hydrocephalus clinical research network quality improvement initiative. J Neurosurg Pediatr. 2011. 8: 22-9
11. Kreutmair S, Kauffmann M, Unger S, Ingelfinger F, Núñez NG, Alberti C. Preexisting comorbidities shape the immune response associated with severe COVID-19. J Allergy Clin Immunol. 2022. 150: 312-24
12. Kulkarni AV, Drake JM, Lamberti-Pasculli M. Cerebrospinal fluid shunt infection: A prospective study of risk factors. J Neurosurg. 2001. 94: 195-201
13. Langel SN, Blasi M, Permar SR. Maternal immune protection against infectious diseases. Cell Host Microbe. 2022. 30: 660-74
14. Mallucci CL, Jenkinson MD, Conroy EJ, Hartley JC, Brown M, Dalton J. Antibiotic or silver versus standard ventriculoperitoneal shunts (BASICS): A multicentre, single-blinded, randomised trial and economic evaluation. Lancet. 2019. 394: 1530-9
15. Parke MA, Perez-Sanchez A, Zamil DH, Katta R, editors. Diet and skin barrier: The role of dietary interventions on skin barrier function. Dermatol Pract Concept. 2021. p. e2021132
16. Ramenghi LA, Fumagalli M, Groppo M, Consonni D, Gatti L, Bertazzi PA. Germinal matrix hemorrhage: Intraventricular hemorrhage in very-low-birth-weight infants. Stroke. 2011. 42: 1889-93
17. Raqib R, Alam DS, Sarker P, Ahmad SM, Ara G, Yunus M. Low birth weight is associated with altered immune function in rural Bangladeshi children: A birth cohort study. Am J Clin Nutr. 2007. 85: 845-52
18. Simon TD, Butler J, Whitlock KB, Browd SR, Holubkov R, Kestle JR. Risk factors for first cerebrospinal fluid shunt infection: Findings from a multi-center prospective cohort study. J Pediatr. 2014. 164: 1462-8.e2
19. Simon TD, Hall M, Riva-Cambrin J, Albert JE, Jeffries HE, LaFleur B. Infection rates following initial cerebrospinal fluid shunt placement across pediatric hospitals in the United States. J Neurosurg Pediatr. 2009. 4: 156-65
20. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016. 355: i4919
21. Stone JJ, Walker CT, Jacobson M, Phillips V, Silberstein HJ. Revision rate of pediatric ventriculoperitoneal shunts after 15 years. J Neurosurg Pediatr. 2013. 11: 15-9
22. Widia Y, Rosdiana BI. Review article: Skin condition and skin care in premature infants. Berkala Ilmu Kesehatan Kulit Kelamin. 2023. 35: 67-73
23. Wong TT, Liang ML, Chen HH, Chang FC. Hydrocephalus with brain tumors in children. Childs Nerv Syst. 2011. 27: 1723-34