- Department of Neurosurgery, Fukuoka Red Cross Hospital, Fukuoka, Japan
- Department of Neurosurgery Fukuoka University Hospital, School of Medicine, Fukuoka University, Fukuoka, Japan.
Correspondence Address:
Naoki Wakuta, Department of Neurosurgery, Fukuoka Red Cross Hospital, Fukuoka, Japan.
DOI:10.25259/SNI_285_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Naoki Wakuta1, Tsutomu Yoshioka1, Yukino Irie1, Hitoshi Tsugu1, Hiroshi Abe2. Ruptured distal anterior inferior cerebellar artery aneurysm years after stereotactic radiosurgery for vestibular schwannoma: A case report and literature review. 21-Jun-2024;15:213
How to cite this URL: Naoki Wakuta1, Tsutomu Yoshioka1, Yukino Irie1, Hitoshi Tsugu1, Hiroshi Abe2. Ruptured distal anterior inferior cerebellar artery aneurysm years after stereotactic radiosurgery for vestibular schwannoma: A case report and literature review. 21-Jun-2024;15:213. Available from: https://surgicalneurologyint.com/surgicalint-articles/12949/
Abstract
Background: Aneurysmal formation after stereotactic radiosurgery (SRS) for vestibular schwannoma (VS) is a rare complication. Its characteristics and the best treatment strategies remain controversial, and the clinical course is especially unknown because reported aneurysms are first incidentally detected, or aneurysmal rupture occurs suddenly, and they are treated immediately.
Case Description: A 68-year-old man who underwent SRS for VS 20 years ago presented with subarachnoid hemorrhage (SAH) due to rupture of a radiation-induced fusiform anterior inferior cerebellar artery aneurysm. He was treated with parent artery occlusion, resulting in a modified Rankin scale grade 2. This report illustrates the first case of detected aneurysm formation before rupture with retrospective magnetic resonance imaging evaluation.
Conclusion: We describe the possible risk of rapid progression and rupture of aneurysms, focusing on the interval from SRS to aneurysmal formation. The period of formation of SRS-induced aneurysms is suspected to vary from years to decades regardless of radiation doses; however, aneurysms estimated as pseudoaneurysms have an extremely high risk of rupture within a few years, even when small in size. If aneurysms are discovered unruptured, there are some advantages in not only the prevention of poor prognosis due to SAH but also in the availability of optional therapeutic strategies using revascularization. Long-term annual follow-up, including vessel examination, is warranted not only to assess tumor status but also for early detection of any vascular lesions.
Keywords: Aneurysm, Endovascular surgery, Stereotactic radiosurgery, Subarachnoid hemorrhage, Vestibular schwannoma
INTRODUCTION
Stereotactic radiosurgery (SRS) is a well-established therapeutic modality for treating vestibular schwannoma (VS). Although radiation-induced vasculopathy is known as a delayed complication after SRS, delayed aneurysm formation is rare, and information on its incidence, clinical course, and the best treatment strategy is lacking. We present here a case of a ruptured distal anterior inferior cerebellar artery (AICA) aneurysm induced by stereotactic irradiation to treat VS and discuss its clinical course and treatment indications with a review of the literature.
CASE PRESENTATION
The patient provided informed consent for the publication of his anonymized data. A 68-year-old man underwent linear accelerator radiotherapy-based SRS of 20 Gy in three fractions for right VS [
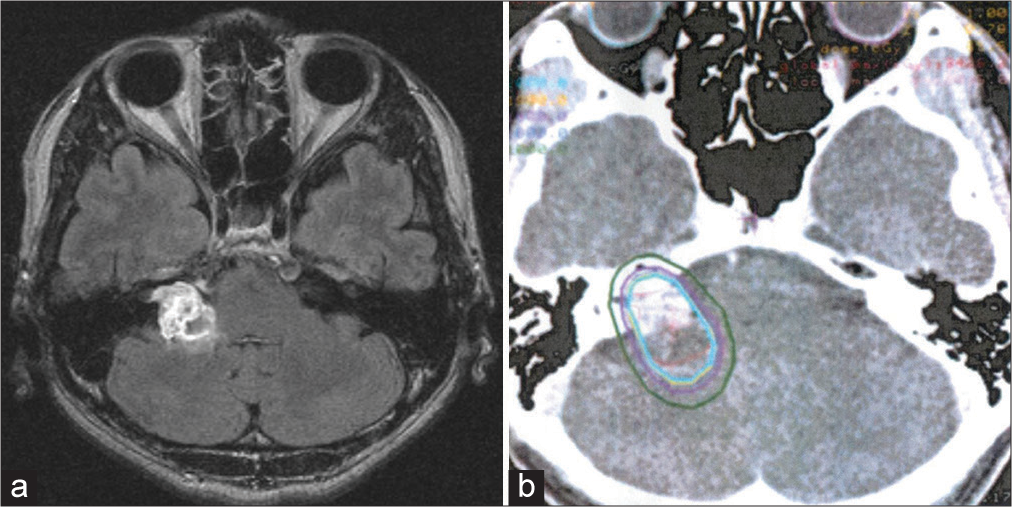
Figure 1:
(a) Magnetic resonance imaging performed before stereotactic radiosurgery showing a vestibular schwannoma and (b) axial dose distribution on a vestibular schwannoma target volume superimposed onto the computed tomography scans. The isodose curves corresponding to 20 Gy (cyan) and 10 Gy (green) are shown.
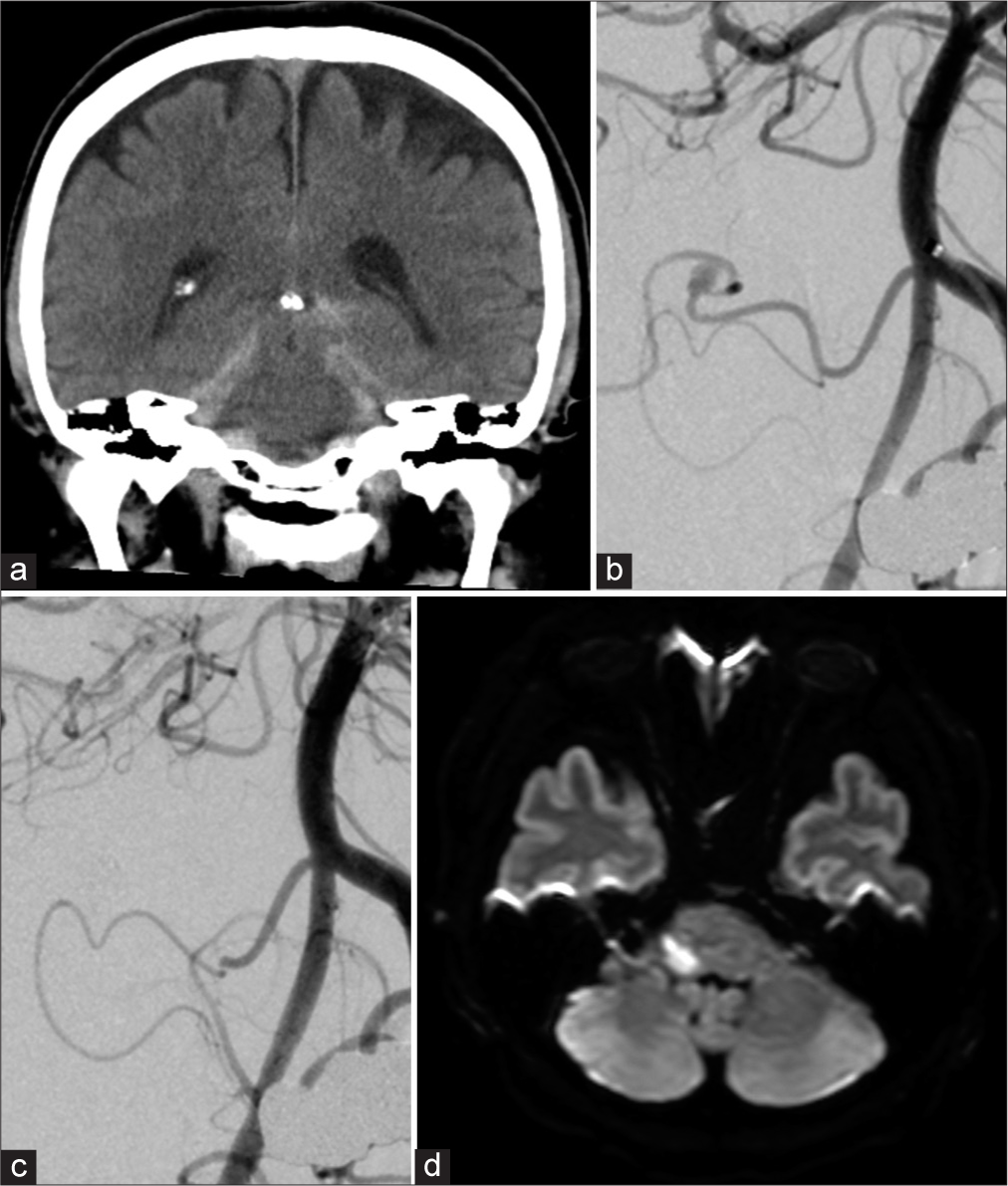
Figure 2:
(a) Computed tomography on admission, (b) left vertebral angiogram showing a fusiform aneurysm of the distal anterior inferior cerebellar artery, (c) angiogram after endovascular aneurysmal coil embolization and parent artery occlusion, and (d) postoperative diffusion-weighted magnetic resonance imaging.
DISCUSSION
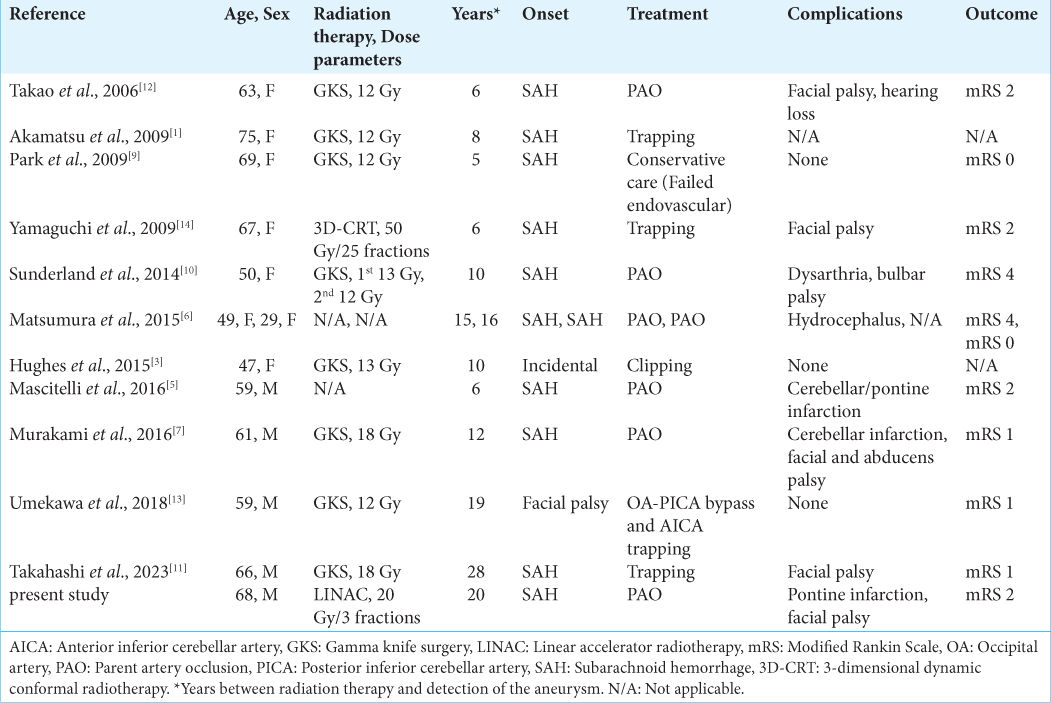
We report here a case of distal AICA aneurysm occurring with SAH 20 years after SRS for VS. Although it is challenging to assess the incidence of such aneurysms, Umekawa et al. estimated the incidence at 0.3% based on data from 360 cases of SRS for VS.[
The pseudoaneurysms were diagnosed using intraoperative findings in five cases[
No definite treatment has been established for distal AICA aneurysms. Open surgical and neuroendovascular interventions are the two main modalities selected for treatment. As the previously reported aneurysms were located at a nonbranching portion of the AICA, endovascular PAO or surgical trapping without revascularization was the most performed treatment in ten of the 13 cases, including the current case [
Open surgical interventions at an irradiated and/or postoperative site are extremely challenging due to adhesions and residual tumor necrosis and carry the risk of aneurysmal rupture or trauma to the nerves. In this review of the literature, all of the aneurysms found during surgical exploration were within the tumor.[
Meanwhile, the necessity of PAO for such ruptured aneurysms was demonstrated in the case reported by Choi et al.[
It would be reasonable to attempt detecting such aneurysms in unruptured states, considering the negligible risk of causing SAH and the availability of surgical options such as extracranial-intracranial bypass. However, the long-term vascular complications after SRS have seldom been studied. Nanney et al. reported that a high radiation dose could be associated with a shorter lag time until aneurysm formation,[
CONCLUSION
Distal AICA pseudoaneurysm formation after SRS for VS is extremely rare, but it carries a high risk of rupture within a few years after occurrence. Endovascular PAO or surgical trapping without revascularization for such aneurysms has the risk of brain stem infarction, but the risk of severe disability is relatively low, and no other therapeutic options may be available in cases of rupture. Because the latency until aneurysm formation after SRS is not fully understood, surgeons must be aware of this complication, and long-term screening is recommended for adjacent vascular structures along with follow-up of tumor status.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Akamatsu Y, Sugawara T, Mikawa S, Saito A, Ono S, Takayama K. Ruptured pseudoaneurysm following gamma knife surgery for a vestibular schwannoma. J Neurosurg. 2009. 110: 543-6
2. Choi CH, Cho WH, Choi BK, Lee SW. Rerupture following endovascular treatment for dissecting aneurysm of distal anterior inferior cerebellar artery with parent artery preservation: Retreatment by parent artery occlusion with Guglielmi detachable coils. Acta Neurochir (Wien). 2006. 148: 363-6
3. Hughes JD, Osetinsky LM, Jacob JT, Carlson ML, Lanzino G, Link MJ. Incidentally discovered unruptured AICA aneurysm after radiosurgery for vestibular schwannoma: A case report and review of the literature. Otol Neurotol. 2015. 36: 1428-31
4. Lorenzoni J, David P, Levivier M. MR-based follow-up of the superior cerebellar artery radiosurgery for trigeminal neuralgia. Clin Neurol Neurosurg. 2011. 113: 758-61
5. Mascitelli JR, Mcneill IT, Mocco J, Berenstein A, Demattia J, Fifi JT. Ruptured distal AICA pseudoaneurysm presenting years after vestibular schwannoma resection and radiation. J Neurointerv Surg. 2016. 8: e19
6. Matsumura H, Kato N, Hosoo H, Fujiwara Y. Subarachnoid hemorrhage due to anterior inferior cerebellar artery aneurysms associated with gamma knife surgery for vestibular schwannomas. Acta Neurochir (Wien). 2015. 157: 1765-7
7. Murakami M, Kawarabuki K, Inoue Y, Ohta T. Ruptured pseudoaneurysm after gamma knife surgery for vestibular schwannoma. Neurol Med Chir (Tokyo). 2016. 56: 38-42
8. Nanney AD, El Tecle NE, El Ahmadieh TY, Daou MR, Bit Ivan EN, Marymont MH. Intracranial aneurysms in previously irradiated fields: literature review and case report. World Neurosurg. 2014. 81: 511-9
9. Park KY, Ahn JY, Lee JW, Chang JH, Huh SK. De novo intracranial aneurysm formation after gamma knife radiosurgery for vestibular schwannoma. J Neurosurg. 2009. 110: 540-2
10. Sunderland G, Hassan F, Bhatnager P, Mitchell P, Jayakrishnan V, Foster D. Development of anterior inferior cerebellar artery pseudoaneurysm after gamma knife surgery for vestibular schwannoma. A case report and review of the literature. Br J Neurosurg. 2014. 28: 536-8
11. Takahashi S, Ishii Y, Aizawa Y, Sato A, Nemoto S. A case of ruptured aneurysm in the internal auditory canal 28 years after gamma knife radiosurgery for a vestibular schwannoma. Surg Cereb Stroke (Japan). 2023. 51: 227-32
12. Takao T, Fukuda M, Kawaguchi T, Nishino K, Ito Y, Tanaka R. Ruptured intracranial aneurysm following gamma knife surgery for acoustic neuroma. Acta Neurochir (Wien). 2006. 148: 1317-8
13. Umekawa M, Hasegawa H, Shin M, Kawashima M, Nomura S, Makatomi H. Radiosurgery-induced anterior inferior cerebellar artery pseudoaneurysm treated with trapping and bypass. World Neurosurg. 2018. 116: 209-13
14. Yamaguchi S, Kato T, Takeda M, Ikeda H, Kitamura K. Ruptured distal anterior inferior cerebellar artery aneurysm following stereotactic irradiation for vestibular schwannoma: Case report. Neurol Med Chir (Tokyo). 2009. 49: 202-5