- Department of Neurosurgery, Aga Khan University Hospital, Karachi, Pakistan
Correspondence Address:
Muhammad E. Bari
Department of Neurosurgery, Aga Khan University Hospital, Karachi, Pakistan
DOI:10.4103/sni.sni_78_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Aleena Khan, Muhammad Waqas, Waseem M. Nizamani, Muhammad E. Bari. Ruptured mycotic aneurysms: Report and outcomes of two surgically managed patients. 11-Jul-2017;8:144
How to cite this URL: Aleena Khan, Muhammad Waqas, Waseem M. Nizamani, Muhammad E. Bari. Ruptured mycotic aneurysms: Report and outcomes of two surgically managed patients. 11-Jul-2017;8:144. Available from: http://surgicalneurologyint.com/surgicalint-articles/ruptured-mycotic-aneurysms-report-and-outcomes-of-two-surgically-managed-patients/
Abstract
Background:Mycotic aneurysm is a rare potentially life-threatening complication of infective endocarditis (IE). Little data is available on the management and outcomes of ruptured mycotic aneurysms with large intracerebral hematoma. Few cases have been described on the management of mycotic aneurysm in the presence of life-threatening hematoma and mass effect.
Case Description:We are presenting two cases of ruptured mycotic aneurysm with intracerebral hematoma and impending brain herniation. Both patients had signs of high intracranial pressure and required urgent surgical evacuation of clot. One patient survived while the other patient expired soon after surgery.
Conclusion:Mycotic aneurysm of middle cerebral artery (MCA) in IE with intracranial hemorrhage is rare and urgent surgical decompression, and aneurysmal clipping can be lifesaving.
Keywords: Infective endocarditis, intracranial hemorrhage, mycotic aneurysm, neurosurgical emergency, rheumatic heart disease
INTRODUCTION
Intracranial mycotic aneurysms are a rare complication of infective endocarditis (IE) associated with profound morbidity and mortality.[
CASE DESCRIPTION
Case 1
Clinical presentation
A 16-year-old female student presented in emergency department (ED) with sudden severe, throbbing right-sided headache followed by an episode of seizure involving jerky movements of left side of her body. Two months earlier, she had been diagnosed with rheumatic heart disease, and was taking antibiotics for IE for 1 month. Although she had not spiked fever in the past 3 days, she had been intermittently febrile for 6 months. Her initial blood cultures had revealed Streptococcus mitis growth.
On examination, she was drowsy, with no eye opening. She was localizing from her right side. Her pupils were equal and reactive. The pulse rate was regular (98 beat per minute) and blood pressure was 122/76 mm Hg. Cardiac auscultation revealed normal first and second heart sounds, a pansystolic murmur in the mitral region and S3 gallop rhythm.
Investigations
At the time of admission, her hemoglobin was 8.9 gm/dL and leukocyte count was elevated (22.7 × 109/mm3). Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were 102 mm/h and 6.2 mg/dL, respectively. Blood cultures were found to grow Streptococcus mitis. Transthoracic echocardiograph showed a severely dilated left atrium, moderate mitral, and tricuspid regurgitation with an echogenic area measuring 12 × 8 mm in size located on mitral valve consistent with vegetation. Electroencephalogram showed asymmetric electrical activity with right-sided suppression.
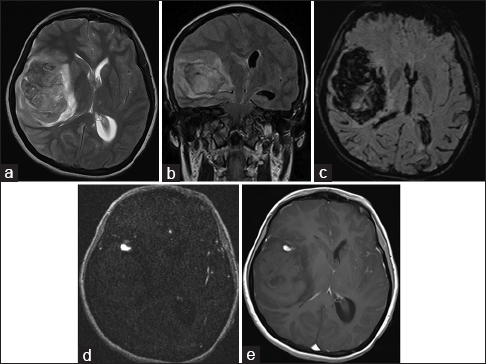
On magnetic resonance imaging (MRI), a large intraparenchymal hematoma was identified in the right temporoparietal region with intraventricular extension, mass effect, and midline shift. A small enhancing focus was seen in M2 segment of right middle cerebral artery (MCA) likely representing mycotic aneurysm [
Treatment
Due to a sudden decrease in responsiveness soon after arrival, she was sedated, intubated, and admitted to the intensive care unit (ICU). She was started on meropenem (1 gram every 8 hours) and vancomycin (1 gram every 12 hours). Over few hours her neurological status got worse. Pupils became anisocoric and motor response dropped to bilateral abnormal extension. Family after much delay consented for surgery. A right frontotemporal craniotomy was performed for clot evacuation. As soon as the duratomy was performed brain tissue started herniating out of bone defect. Normal parenchyma of frontal and temporal lobe had to be resected along with clot evacuation to enable wound closure. A fusiform aneurysm was noticed in M3 part of MCA which we left unclipped as the patient did not seem salvageable. Wound was closed rapidly without bone flap. She was shifted to ICU where she did not show any improvement. Family was explained poor prognosis and ventilator support was withdrawn gradually.
Outcome and follow-up
Patient expired over next 24 hours.
Case 2
Clinical presentation
A 30-year-old male presented in ED with right hemiparesis and slurred speech for 1 day. He had been intermittently febrile for the past 5–6 months and was diagnosed with IE 6 weeks prior to presentation, for which he was receiving intravenous antibiotics.
On examination, he was afebrile. His heart rate was 100 beats per minute (bpm) and blood pressure was 100/50 mm Hg. He had mild clubbing (Grade 1), with an otherwise unremarkable general examination. He was drowsy, disoriented, and unable to follow commands. He had eye opening to command and localized from his left side.
Investigations
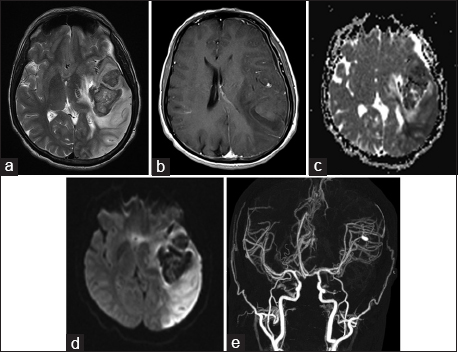
Initial blood work up showed hemoglobin of 11.6 gm/dL. Leukocyte count was elevated (i.e., 13.9 × 109/mm3). Blood cultures did not grow any organism. ESR was 79. Transthoracic echocardiography showed vegetations on the aortic valve measuring 22 mm × 13 mm and mitral valve measuring 20 mm × 11 mm. Preliminary MRI suggested acute hemorrhagic infarction of left MCA with significant cerebral edema, midline shift, and mass effect. CT angiogram of brain exhibited a fusiform aneurysm of posterior sylvian branch of left MCA [
Treatment
Initially, a conservative approach was employed with administration of antibiotics (meropenem 1 gram every 8 hours and vancomycin 1 g every 12 hours). On second day of admission we observed a drop in motor and verbal response. Glasgow coma scale dropped from 10 to 8. He was rushed to theatre immediately where frontotemporal decompressive craniectomy was done, clot evacuated and aneurysm of M3 clipped. Postoperatively he was shifted to ICU where he started showing gradual improvement. Over a week he started obeying commands and started moving left side. Cranioplasty was performed after 3 months. He was managed with a multidisciplinary approach with cardiothoracic surgery and infectious disease department.
Outcome and follow-up
Good recovery with mild residual disability was noted at 6 months follow-up.
DISCUSSION
Symptomatic neurological manifestations are seen in 35% of IE patients.[
We have described two cases of ruptured mycotic aneurysm with large intracerebral hematomas causing immediate threat to life. A review of literature revealed that rupture rates vary from <2% to 72%.[
These cases are unique in several respects. Allen et al. have presented the largest case series of mycotic aneurysms so far with 26 patients.[
There are several other case reports of mycotic aneurysms, but very few have described ruptured mycotic aneurysm with impending herniation.
In a series by Kannoth et al. 10 patients had mycotic aneurysm due to IE.[
Mycotic aneurysms rarely present with subdural hematoma as seen in our case 2. Only 10 such cases have been reported.[
Antibiotics are the mainstay of treatment of unruptured mycotic aneurysms. Literature supports a minimum at 4–6 weeks of antibiotic therapy with serial CT scans and angiography to follow the status of the mycotic aneurysm.[
CONCLUSION
Mycotic aneurysm of MCA in the setting of IE with intraparenchymal hemorrhage and/or subdural hematoma is rare. An urgent angiogram is warranted in any IE patient presenting with neurological symptoms. If performed timely urgent surgical decompression and aneurysmal clipping can be lifesaving for cases with impending herniation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Allen L, Fowler A, Walker C, Derdeyn C, Nguyen B, Hasso A. Retrospective review of cerebral mycotic aneurysms in 26 patients: Focus on treatment in strongly immunocompromised patients with a brief literature review. Am J Neuroradiol. 2013. 34: 823-7
2. Ducruet AF, Hickman ZL, Zacharia BE, Narula R, Grobelny BT, Gorski J. Intracranial infectious aneurysms: A comprehensive review. Neurosurg Rev. 2010. 33: 37-46
3. Frazee JG, Cahan LD, Winter J. Bacterial intracranial aneurysms. J Neurosurg. 1980. 53: 633-41
4. Huang J, McGirt MJ, Gailloud P, Tamargo RJ. Intracranial aneurysms in the pediatric population: Case series and literature review. Surg Neurol. 2005. 63: 424-32
5. Kannoth S, Thomas SV. Intracranial microbial aneurysm (infectious aneurysm): Current options for diagnosis and management. Neurocrit Care. 2009. 11: 120-9
6. Kundra SN. Management of intracranial infectious aneurysms: A series of 16 cases. Neurosurgery. 2003. 53: 245-6
7. Peters PJ, Harrison T, Lennox JL. A dangerous dilemma: Management of infectious intracranial aneurysms complicating endocarditis. Lancet Infect Dis. 2006. 6: 742-8
8. Phuong LK, Link M, Wijdicks E. Management of intracranial infectious aneurysms: A series of 16 cases. Neurosurgery. 2002. 51: 1145-52
9. Snygg-Martin U, Gustafsson L, Rosengren L, Alsiö Å, Ackerholm P, Andersson R. Cerebrovascular complications in patients with left-sided infective endocarditis are common: A prospective study using magnetic resonance imaging and neurochemical brain damage markers. Clin Infect Dis. 2008. 47: 23-30