- Department of Neurosurgery, Japan Community Health Care Organization (JCHO) Chukyo Hospital, Nagoya, Japan.
- Department of Neurosurgery, Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital, Nagoya, Japan.
Correspondence Address:
Yusuke Sakamoto, Department of Neurosurgery, Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital, Nagoya, Japan.
DOI:10.25259/SNI_344_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Daiki Somiya1, Yusuke Sakamoto2, Kenko Maeda1, Syuntaro Takasu1, Masaya Takemoto1, Jungsu Choo1, Mizuka Ikezawa1, Fumihiro Sago1, Kohei Doba1, Akira Ikeda1. Ruptured proximal middle cerebral artery traumatic pseudoaneurysm treated with bypass-assisted trapping surgery: A case report. 28-Jul-2023;14:263
How to cite this URL: Daiki Somiya1, Yusuke Sakamoto2, Kenko Maeda1, Syuntaro Takasu1, Masaya Takemoto1, Jungsu Choo1, Mizuka Ikezawa1, Fumihiro Sago1, Kohei Doba1, Akira Ikeda1. Ruptured proximal middle cerebral artery traumatic pseudoaneurysm treated with bypass-assisted trapping surgery: A case report. 28-Jul-2023;14:263. Available from: https://surgicalneurologyint.com/surgicalint-articles/12467/
Abstract
Background: Traumatic pseudoaneurysms are rare but have a high mortality rate; therefore, immediate diagnosis is vital. Most pseudoaneurysms are in the internal carotid artery or peripheral arteries, while proximal middle cerebral artery pseudoaneurysms have rarely been reported. We present a case of ruptured traumatic pseudoaneurysm located at the M1-M2 bifurcation.
Case Description: A 42-year-old man was injured in a motorcycle accident and his Glasgow coma scale score on admission was 7 (Eye opening1, Verbal response2, Motor response4 [E1V2M4]). Head computed tomography (CT) showed thick subarachnoid hemorrhage (SAH). We suspected a ruptured aneurysm, but three-dimensional CT angiography (3D-CTA) did not detect any vascular defects. Head magnetic resonance angiography showed progressive right M1 stenosis suggesting arterial dissection. 3D-CTA on day 20 showed a small aneurysm in the proximal portion of the M2. Although surgery was scheduled for day 26, suddenly left hemiparesis appeared on day 24. Head CT detected fresh SAH and emergency surgery was performed on day 25. We dissected around the ruptured point under M1 temporary occlusion with superficial temporal artery-M2 assist bypass. Contrary to our expectations, there was only a small laceration in the right M2 superior trunk. We trapped the laceration and the proximal portion of the M2 superior trunk while preserving antegrade blood flow from the M1 to the M2 inferior trunk. On the 5-month follow-up, the patient was able to walk independently.
Conclusion: Unreasonably thick traumatic SAH or spastic stenosis after head injury may indicate a traumatic pseudoaneurysm and require repeated neurovascular evaluation. If a pseudoaneurysm is detected, immediate surgical intervention is mandatory.
Keywords: Bypass, Middle cerebral artery, Trapping surgery, Traumatic aneurysm
INTRODUCTION
Traumatic pseudoaneurysms are rare, which accounting for <1% of cerebral aneurysms, but have a high mortality rate.[
CASE DESCRIPTION
History and diagnosis
Written informed consent for the publication of data and any related images was obtained from the patient.
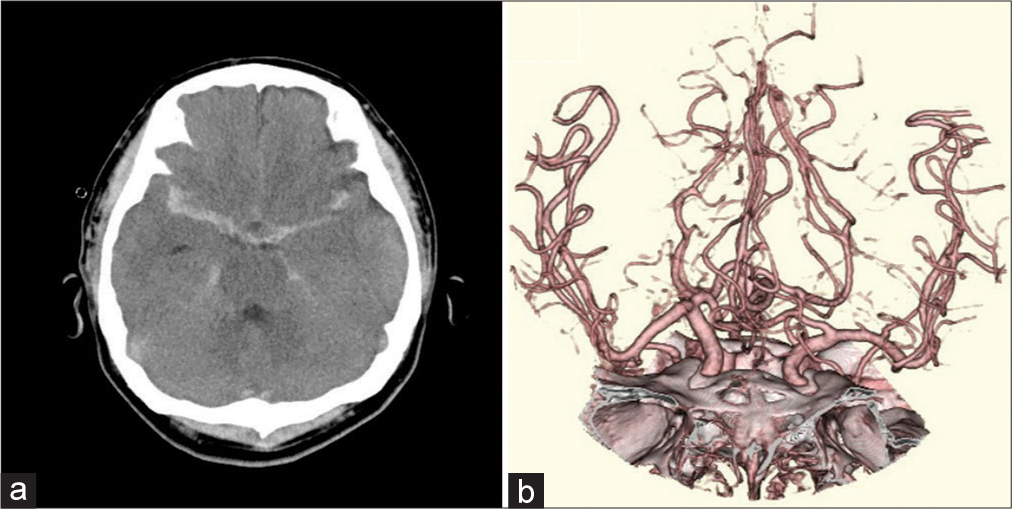
A 42-year-old man was admitted to our hospital due to multiple injury sustained in a motorcycle accident. The Glasgow coma scale (GCS) score on admission was 7 (E1V2M4). Head computed tomography (CT) revealed thick subarachnoid hemorrhage (SAH) in the right Sylvian fissure [
Follow-up head magnetic resonance imaging (MRI) and diffusion-weighted imaging performed on day 7 showed small high-intensity areas in the bilateral hemispheres and corpus callosum, indicating diffuse axonal injury. Head magnetic resonance angiography (MRA) showed stenosis of the right M1 portion, suggesting traumatic SAH-induced vasospasm [
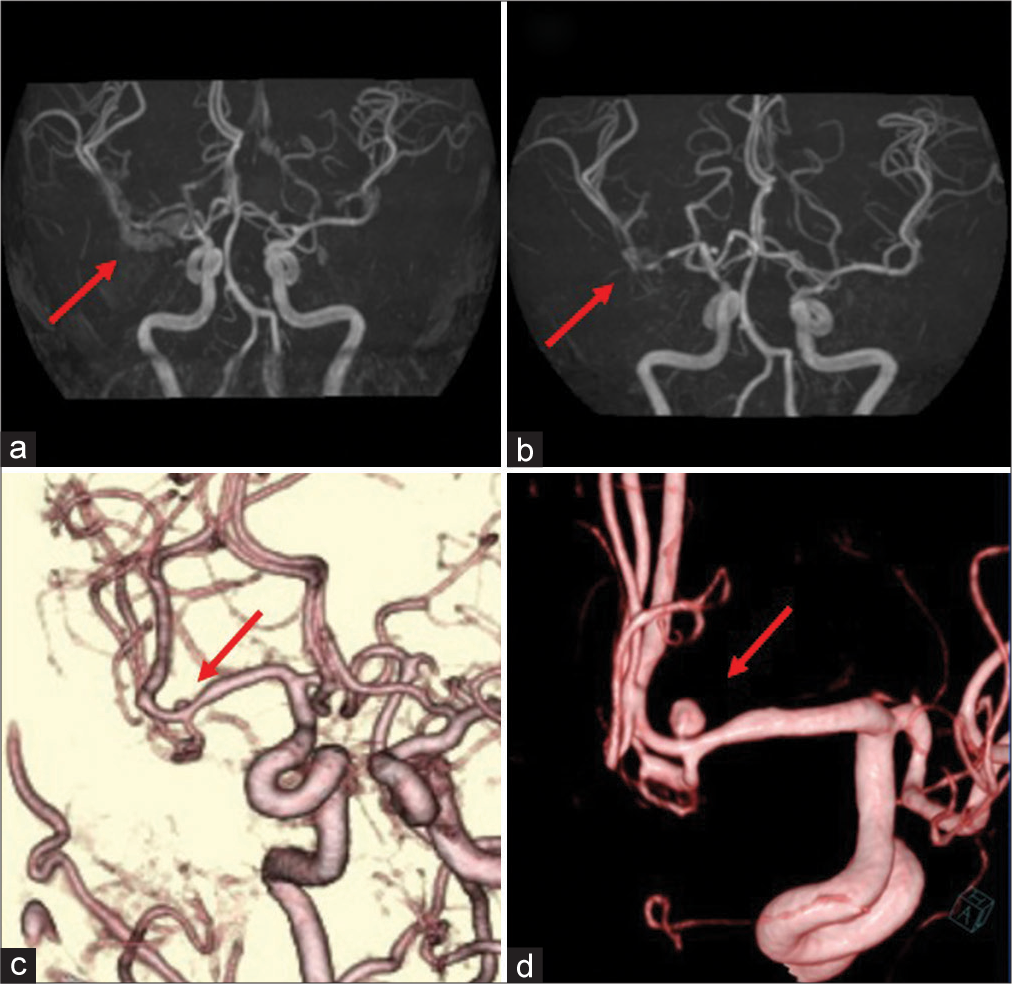
Figure 2:
Follow-up preoperative imaging findings. (a) Magnetic resonance angiography image obtained on day 7 showing mild stenosis of the right M1 portion (arrow). (b) Follow-up magnetic resonance angiography image obtained on day 13 showing progression of the stenosis from M1 to the M1-M2 bifurcation (arrow). (c) Three-dimensional computed tomography angiography image obtained on day 20 showing a small 2.5-mm saccular aneurysm on the superior wall of the M2 superior trunk (arrow). (d) Three-dimensional digital subtraction angiography image obtained on day 23 showing enlargement of the saccular aneurysm (arrow).
On day 13, we performed follow-up MRA, which showed stenosis of the M1 portion extending to the M1-M2 bifurcation [
Surgical treatment was scheduled for day 26; however, the patient suddenly developed left hemiparesis on day 24. Head CT detected fresh SAH around the right Sylvian fissure. We suspected pseudoaneurysm rupture; therefore, we sedated and intubated the patient. Emergency surgery was performed on day 25.
Surgical strategy and treatment
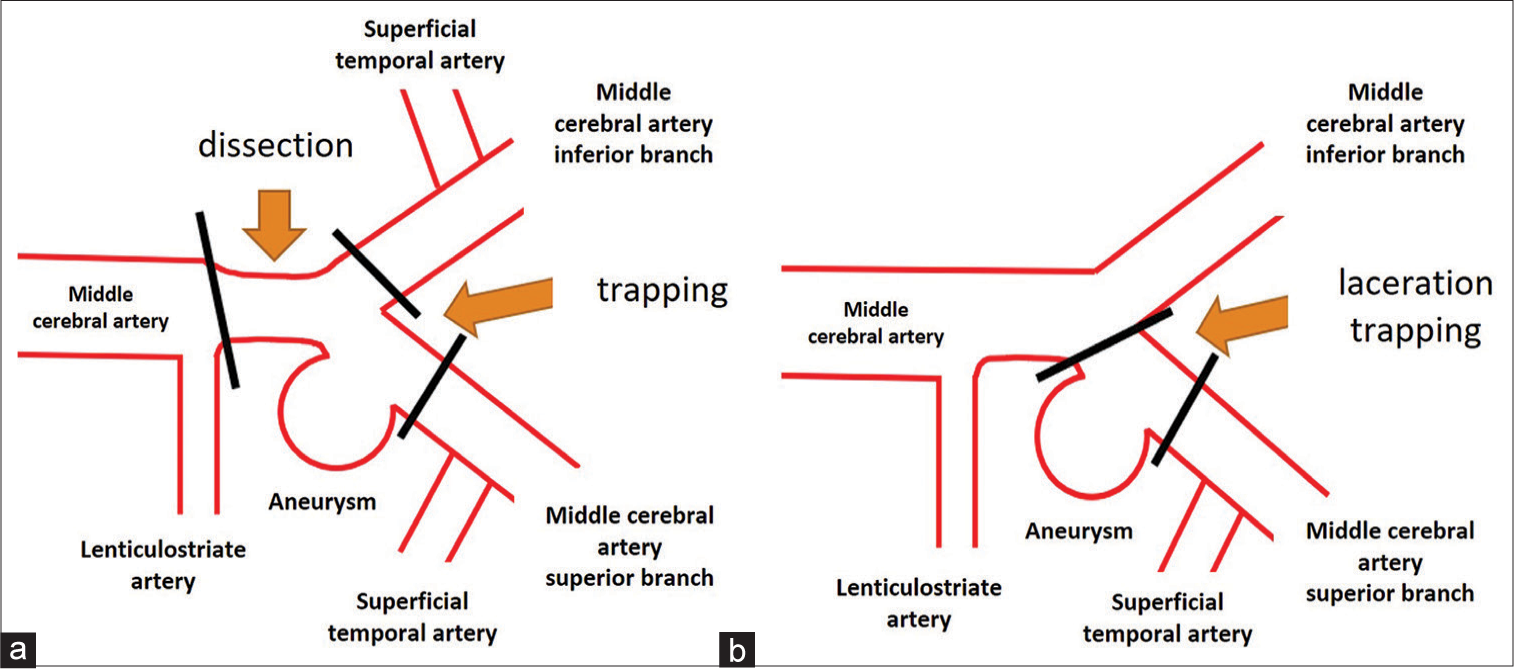
The actual length of the dissecting lesion was uncertain, but we suspected that the stenosis from the right M1 portion to the M1-M2 bifurcation reflected the dissecting lesion and that the left hemiparesis was induced by lenticulostriate artery (LSA) thrombosis caused by M1 dissection. Based on this, we considered M1-M2 trapping and vascular reconstruction. Fortunately, the frontal and parietal branches of the right superficial temporal artery (STA) were well-developed and double-barrel low-flow bypass seemed to be sufficient for reconstruction of the M2 lesion. Hence, we decided to perform M1-M2 trapping surgery assisted by an STA-MCA double-barrel bypass [
Figure 3:
Schematic presentation of the planned and the actual surgical strategies. (a) Preoperatively, middle cerebral artery M1-M2 trapping surgery assisted by superficial temporal artery-middle cerebral artery double-barrel bypass was planned. (b) As the laceration was limited to the superior wall of the M2 superior trunk, M2 clipping was performed assisted by superficial temporary artery-M2 superior trunk bypass to trap the lacerated point and preserve antegrade blood flow from M1 to the M2 inferior trunk. Despite the mild stenosis at the origin of the M2 inferior trunk, superficial temporal artery-M2 inferior trunk bypass was not performed.
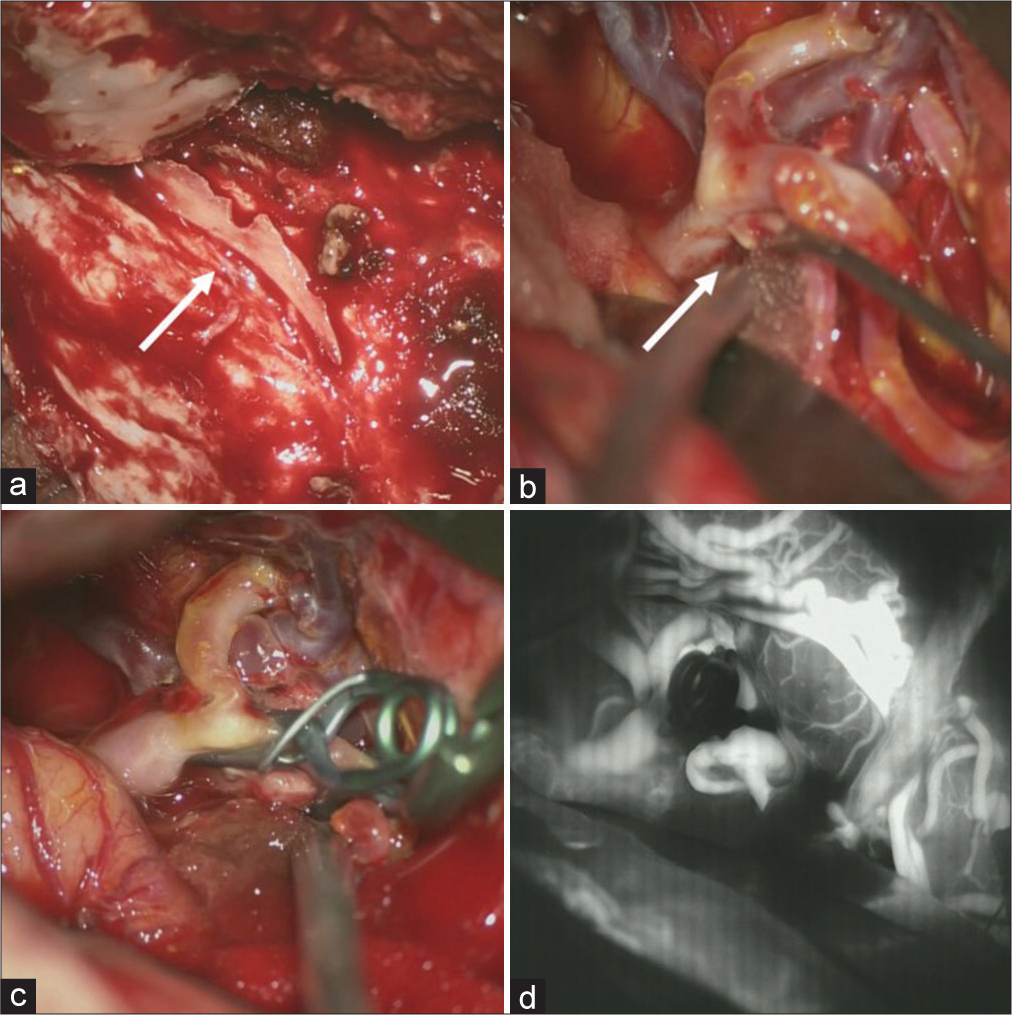
Surgery was performed under general anesthesia with monitoring of somatosensory and motor evoked potentials of the extremities. Both STA branches were harvested and frontotemporal craniotomy was performed in the usual fashion. When we removed the cranial bone, we found a hard bony process attached the surface of the dura mater along the middle meningeal artery [
Figure 4:
Intraoperative findings. (a) A hard bony process is seen (arrow) attached on the surface of the dura matter along the middle meningeal artery. (b) A small laceration on the superior wall of the M2 superior trunk is observed, indicating pseudoaneurysm (arrow). (c) Trapping of the laceration point with preservation of antegrade blood flow from M1 to the M2 inferior trunk assisted by superficial temporal artery-M2 (superior trunk) bypass, despite the mild stenosis of the origin of the M2 inferior trunk. (d) Indocyanine green video angiography image showing perfect patency of the superficial temporal artery-M2 bypass, antegraded blood flow from M1 to the M2 inferior trunk and complete obliteration of the laceration on the M2 superior trunk.
Postoperative course
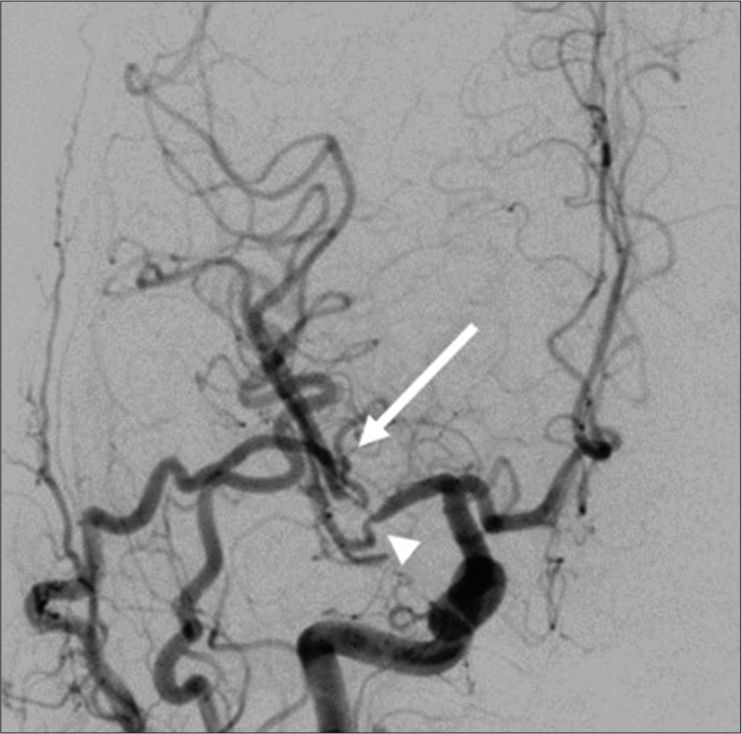
We administered antiplatelet therapy with ozagrel sodium (80 mg/day), edaravone (60 mg/day), fasudil hydrochloride hydrate (90 mg/day), and cilostazol (200 mg/day) for 2 weeks. Follow-up head MRI/A on day 39 showed no additional infarctions. Postoperative DSA on day 56 showed excellent bypass patency with a mild stenotic lesion at the M1-M2 bifurcation and M2 inferior trunk [
DISCUSSION
Traumatic cerebral pseudoaneurysms are very rare but have a high mortality rate of up to 50%.[
In our case, head CT showed SAH predominantly in the right Sylvian fissure. In addition, the patient had severe consciousness disturbance compared to the degree of cerebral contusion. Therefore, we considered the possibility that endogenous SAH may have caused the consciousness disturbance and induced the traffic accident. However, no vascular abnormalities were observed on CTA. Head MRA on day 7 did not show a cerebral aneurysm but detected progression of the stenosis from the right M1 to M2. Concerning about the possibility of SAH due to MCA dissection, we performed repeated neurovascular evaluation. CTA performed on day 20 confirmed the appearance of an aneurysm on the right MCA. The previous studies have also reported difficulty in diagnosing traumatic cerebral pseudoaneurysms[
Traumatic cerebral aneurysms have a higher rupture rate than normal cerebral aneurysms because of their fragility; therefore, early therapeutic intervention is necessary when they are detected.[
Various treatment options have been reported for traumatic cerebral aneurysms, including endovascular treatment.[
Traumatic cerebral aneurysms are common in the ICA and vertebral arteries and have also been reported in the cortical branches of the anterior cerebral artery and MCA.[
CONCLUSION
In cases of unreasonably thick SAH or extensive cerebral vasospasm after head trauma, continuous neurovascular evaluation is mandatory to detect late emergence of traumatic pseudoaneurysms, even if no vascular abnormalities are detected in the primary neurovascular evaluation. Immediate surgical intervention is necessary once a traumatic cerebral aneurysm is confirmed.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Buckingham MJ, Crone KR, Ball WS, Tomsick TA, Berger TS, Tew JM. Traumatic intracranial aneurysms in childhood: Two cases and a review of the literature. Neurosurgery. 1988. 22: 398-408
2. Burton C, Velasco F, Dorman J. Traumatic aneurysm of a peripheral cerebral artery. Review and case report. J Neurosurg. 1968. 28: 468-74
3. Cohen JE, Gomori JM, Segal R, Spivak A, Margolin E, Sviri G. Results of endovascular treatment of traumatic intracranial aneurysms. Neurosurgery. 2008. 63: 476-86
4. Holmes B, Harbaugh RE. Traumatic intracranial aneurysms: A contemporary review. J Trauma. 1993. 35: 855-60
5. Larson PS, Reisner A, Morassutti DJ, Abdulhadi B, Harpring JE. Traumatic intracranial aneurysms. Neurosurg Focus. 2000. 8: e4
6. Niu Y, Zhou S, Tang J, Miao H, Zhu G, Chen Z. Treatment of traumatic intracranial aneurysm: Experiences at a single center. Clin Neurol Neurosurg. 2020. 189: 105619
7. Ohta M, Matsuno H. Proximal M2 false aneurysm after head trauma--case report. Neurol Med Chir (Tokyo). 2001. 41: 131-4
8. Parkinson D, West M. Traumatic intracranial aneurysms. J Neurosurg. 1980. 52: 11-20
9. Rumbaugh CL, Bergeron RT, Talalla A, Kurze T. Traumatic aneurysms of the cortical cerebral arteries. Radiographic aspects. Radiology. 1970. 96: 49-54
10. Shi Y, Gao Y, Liu Y, Cui W, Zhou G, Wang L. Treatment of traumatic intracranial pseudoaneurysms: A single-center experience. Front Neurol. 2021. 12: 690284
11. Smith DR, Kempe LG. Cerebral false aneurysm formation in closed head trauma; case report. J Neurosurg. 1970. 32: 357-9
12. Voelker JL, Ortiz O. Delayed deterioration after head trauma due to traumatic aneurysm. W V Med J. 1997. 93: 317-9
13. Zheng Y, Lu Z, Shen J, Xu F. Intracranial pseudoaneurysms: Evaluation and management. Front Neurol. 2020. 11: 582