- Department of Neurosurgery, Baptist Neurological Institute, Jacksonville, Florida, United States
- Department of Neurosurgery, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HC-FMUSP), Sao Paulo, Brazil
Correspondence Address:
Eberval Gadelha Figueiredo, MD, PhD, Department of Neurosurgery, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HC-FMUSP), São Paulo, Brazil.
DOI:10.25259/SNI_365_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Douglas Gonsales1, Eberval Gadelha Figueiredo2, Joao Paulo Mota Telles2, Pedro Aguilar-Salinas1, Nima Amin Aghaebrahim1, Eric Sauvageau1, Saul Almeida da Silva2, Ricardo A. Hanel1. Safety and efficacy of mechanical thrombectomy for acute ischemic stroke with volume over 50 mL and significant perfusion mismatch. 30-Aug-2024;15:308
How to cite this URL: Douglas Gonsales1, Eberval Gadelha Figueiredo2, Joao Paulo Mota Telles2, Pedro Aguilar-Salinas1, Nima Amin Aghaebrahim1, Eric Sauvageau1, Saul Almeida da Silva2, Ricardo A. Hanel1. Safety and efficacy of mechanical thrombectomy for acute ischemic stroke with volume over 50 mL and significant perfusion mismatch. 30-Aug-2024;15:308. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13073
Abstract
Background: This study aims to address the safety and efficacy of mechanical thrombectomy (MT) in acute ischemic stroke with an established infarction equal to or >50 mL with a significant difference between penumbra and established infarction detected by perfusion cerebral computed tomography (CT) with the Rapid® system.
Methods: This was a retrospective case–control study. Patients diagnosed with established and extensive ischemic stroke, defined by an ischemic volume equal to or >50 mL on CT or magnetic resonance imaging perfusion using the RAPID® system, were examined. The intervention group received endovascular interventional treatment with or without recombinant tissue plasminogen activator (rt-PA) in addition to standard therapy, and the control group received conservative treatment with or without rt-PA plus standard therapy.
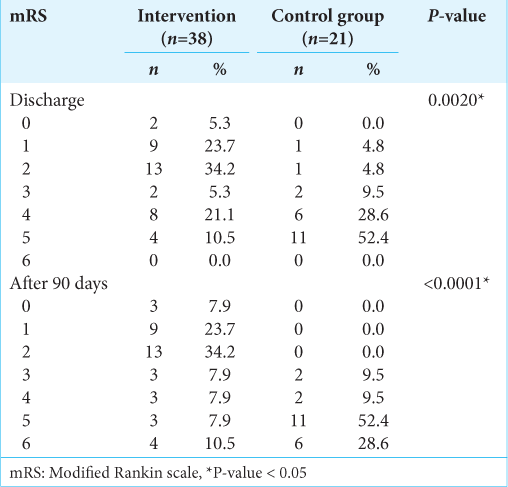
Results: A total of 59 patients were enrolled, including 38 in the intervention group and 21 in the control group. Baseline characteristics were similar between groups. Patient National Institutes of Health Stroke Scale at discharge was significantly different between the control (median 30, interquartile range [IQR] 13) and intervention group (median 8, IQR 14) (P P = 0.002). These mRS differences remained significant at 90 days, with median (IQR) values of 2 (2.75) and 5 (1), respectively (P
Conclusion: MT is safe and effective for large-core ischemic strokes with significant perfusion mismatch, leading to better functional outcomes without significant complications compared to the best medical treatment.
Keywords: Ischemic stroke, Reperfusion, Stroke, Thrombectomy, Endovascular
INTRODUCTION
Ischemic stroke remains a major global cause of morbidity and mortality despite advancements in medical therapy.[
In this evolving landscape, recent clinical trials such as RESCUE-Japan LIMIT, SELECT2, and ANGEL-ASPECT2 have provided significant insights into the use of MT for large-core ischemic strokes.[
This study aims to address the safety and efficacy of MT in acute ischemic stroke with an established infarction equal to or >50 mL with a significant difference between penumbra and established infarction detected by perfusion cerebral computed tomography (CT) with the Rapid® system.
MATERIALS AND METHODS
This study was a retrospective case–control. Patients diagnosed with established and extensive ischemic stroke, defined by an ischemic volume equal to or >50 mL on CT or magnetic resonance imaging (MRI) perfusion using the RAPID® system, were examined. Two groups were studied: One group received endovascular interventional treatment with or without recombinant tissue plasminogen activator (rt-PA) in addition to standard therapy, and the other group received conservative treatment with or without rt-PA plus standard therapy. Data collection occurred at the Baptist Medical Center in Jacksonville, FL, USA, from January 2016 to December 2019. The Local Institutional Review Board approved the study (study 16–58 of the North Florida Stroke Patient Registry).
All patients received pre-hospital care from a highly trained team, either by ground or air, arriving at the Baptist Medical Center’s emergency department. A code stroke was declared when a possible cerebral ischemia/hemorrhage was suspected, at which point a nurse practitioner from the interventional neurosurgery department would conduct a clinical assessment (National Institutes of Health Stroke Scale [NIHSS] and modified Rankin scale [mRS]) and immediately send the patient for imaging, including unenhanced CT, perfusion CT, and brain AngioCT. The Rapid® system was used to analyze all data and images, alerting the team through a mobile application when a Code Stroke was declared. The team would then assess whether endovascular intervention was warranted.
Inclusion and exclusion criteria
Inclusion criteria were patients over 18 years old with acute occlusion of the internal carotid artery, middle cerebral artery, anterior cerebral artery, or vertebrobasilar system. Patients with a baseline mRS 0–2 were included in the study. NIHSS equal to or >5. Symptom onset up to 24 h or unknown. CT or MRI images for ASPECTS evaluation and collaterals were evaluated by two independent neuroradiologists blind to patient information.
Exclusion criteria included patients with a cerebral infarct volume equal to oligemia, pregnancy, suspicion of intracranial arterial dissection, inability to follow-up for mRS at 90 days, and patients with severe diseases that interfere with patient management and follow-up.
Data analysis
Descriptive analyses were used to describe and characterize the two groups: the intervention group (n = 38) and the control group (n = 21). The normality of distribution was assessed using a histogram and the Shapiro-Wilk test. Continuous variables were presented as mean and SD or median and interquartile range (IQR) (if not normally distributed). Categorical variables were presented as numbers and frequencies. Clinical and imaging variables between the two groups were compared using the χ2 test (Categorical variable) or G-test when npq <5. The Mann-Whitney U-test was used to compare non-normally distributed quantitative variables. For text similarity analysis, the Iramuteq version 7.0 program was used,[
RESULTS
Between January 2016 and December 2019, a total of 38 cases required endovascular intervention through thrombectomy, and 21 cases were treated conservatively on patients with ischemic stroke with an established infarct volume equal to or >50 mL, based on cranial CT or MRI perfusion using the RAPID® system.
Baseline characteristics
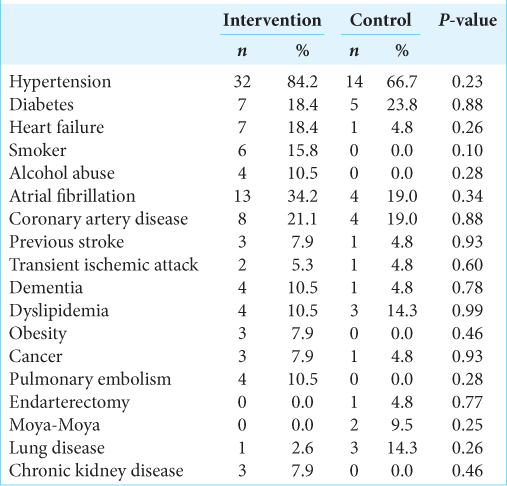
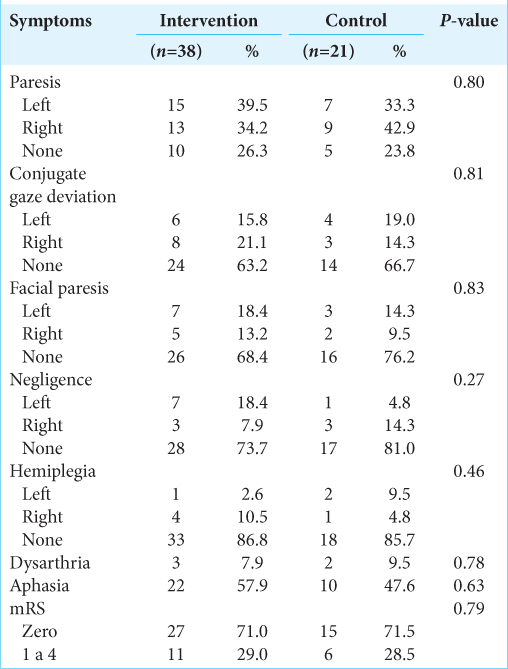
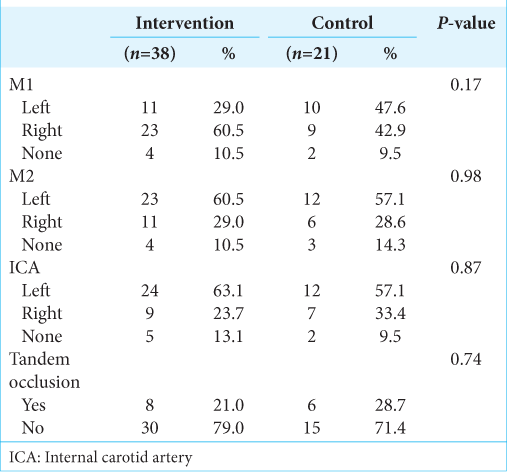
Baseline characteristics are described in
In the control group, there were 9 male patients (42.9%) and 12 female patients (57.1%) with a mean age of 68.9 ± 17.5 years and a median age of 73 years (50–89). In 47.6% of the cases, a left middle cerebral artery syndrome (M1 segment) occurred. The mean NIHSS at admission was 22.0 (0–28). The mean pre-intervention definitive cerebral infarct volume was 97.0 mL (52–281.3 mL), and infarct with oligemia averaged 193.0 mL (65–423 mL). The mismatch between the total volumes that could potentially be preserved (penumbra) was 94 mL (12– 326 mL). The mean ASPECTS score on CT or MRI was 4 (2– 9).
Treatment and outcomes
In the intervention group, thrombectomy with aspiration using a stent retriever was performed in 100% of cases, achieving Thrombolysis in Cerebral Infarction (T1C1) 2b-3 in 84.2% of cases. The rate of mRS 0–2 at 90 days was 65.8%, and the 90-day mortality rate was 10.5%. There was one case of non-significant hemorrhagic transformation, which required surgical treatment.
In the control group, the treatment was the optimization of clinical parameters in a neurological intensive care unit, followed by rehabilitation center treatment. The rate of mRS 0–2 at 90 days was 0%, and the 90-day mortality rate was 28.6%. There were ten cases of cerebral ischemic enlargement, and all the patients were discharged to an inpatient rehabilitation facility for clinical treatment.
Figure 2:
mRS at 90 days. The bar plots show the distribution of mRS scores reevaluated at 3 months. The upper bar represents the intervention group, and the lower bar represents the control group (best medical treatment). The difference was statistically significant (P < 0.001). mRS: Modified Rankin scale.
DISCUSSION
The present study aimed to evaluate the results of MT for large-core ischemic strokes with significant perfusion mismatch. The results demonstrated a significant benefit in terms of functional outcomes without a significant difference in unfavorable complication rates.
Prior studies such as MR CLEAN,[
Our analysis reveals superior outcomes for patients undergoing MT, especially when performed within 6 h of symptom onset, despite the acceptance of endovascular intervention up to 24 h as indicated by the DAWN trial.[
In 2017, Rebello et al.[
Interestingly, patients with an established infarction (core) equal to or >50 mL are not frequently studied. However, our study has shown evidence of MT benefits for patients at this infarction level. Often, patients with this level of established infarction present with smaller penumbral areas, creating a mismatch between the potential salvageable brain tissue and the established infarction.[
While the study offers valuable insights, it is not without limitations, including its retrospective nature, non-randomization, the relatively small sample size in both intervention and control groups, and the lack of standardized protocol for collateral circulation assessment. Nevertheless, it also presents numerous strengths, the most important of which is the application of MT for large-core strokes in a real-life setting.
CONCLUSION
MT is safe and effective for large-core ischemic strokes with significant perfusion mismatch, leading to better functional outcomes without significant complications compared to the best medical treatment.
Ethical approval
The research/study was approved by the Institutional Review Board at North Florida Stroke Patient Registry, number 16-85, dated November 15, 2018.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Berkhemer OA, Fransen PS, Beumer D, Van den Berg LA, Lingsma HF, Yoo AJ. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015. 372: 11-20
2. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015. 372: 1009-18
3. Desilles JP, Consoli A, Redjem H, Coskun O, Ciccio G, Smajda S. Successful reperfusion with mechanical thrombectomy is associated with reduced disability and mortality in patients with pretreatment diffusion-weighted imaging-alberta stroke program early computed tomography score ≤6. Stroke. 2017. 48: 963-9
4. Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet. 2014. 384: 1929-35
5. Feigin VL, Krishnamurthi RV, Parmar P, Norrving B, Mensah GA, Bennett DA. Update on the global burden of ischemic and hemorrhagic stroke in 1990-2013: The GBD 2013 Study. Neuroepidemiology. 2015. 45: 161-76
6. Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: A systematic review. Lancet Neurol. 2009. 8: 355-69
7. Goyal M, Menon BK, Van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet. 2016. 387: 1723-31
8. Huo X, Ma G, Tong X, Zhang X, Pan Y, Nguyen TN. Trial of endovascular therapy for acute ischemic stroke with large infarct. N Engl J Med. 2023. 388: 1272-83
9. Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: Findings from the global burden of disease study 2010. Lancet Glob Health. 2013. 1: e259-81
10. Mosconi MG, Paciaroni M. Treatments in ischemic stroke: Current and future. Eur Neurol. 2022. 85: 349-66
11. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P. Thrombectomy 6 to 24 Hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018. 378: 11-21
12. Rebello LC, Bouslama M, Haussen DC, Dehkharghani S, Grossberg JA, Belagaje S. Endovascular treatment for patients with acute stroke who have a large ischemic core and large mismatch imaging profile. JAMA Neurol. 2017. 74: 34
13. Sarraj A, Hassan AE, Abraham MG, Ortega-Gutierrez S, Kasner SE, Hussain MS. Trial of endovascular thrombectomy for large ischemic strokes. N Engl J Med. 2023. 388: 1259-71
14. Sarraj A, Hassan AE, Savitz S, Sitton C, Grotta J, Chen P. Outcomes of endovascular thrombectomy vs medical management alone in patients with large ischemic cores. JAMA Neurol. 2019. 76: 1147
15. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015. 372: 2285-95
16. Yoshimura S, Sakai N, Yamagami H, Uchida K, Beppu M, Toyoda K. Endovascular therapy for acute stroke with a large Ischemic Region. N Engl J Med. 2022. 386: 1303-13