- Department of Neurosurgery, Baylor College of Medicine/Section of Pediatric Neurosurgery, Texas Children's Hospital, Texas, USA
Correspondence Address:
Sandi Lam
Department of Neurosurgery, Baylor College of Medicine/Section of Pediatric Neurosurgery, Texas Children's Hospital, Texas, USA
DOI:10.4103/2152-7806.205268
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kathryn M. Wagner, Jeffrey S. Raskin, Daniel Hansen, Gaddum D. Reddy, Andrew Jea, Sandi Lam. Surgical management of lipomyelomeningocele in children: Challenges and considerations. 26-Apr-2017;8:63
How to cite this URL: Kathryn M. Wagner, Jeffrey S. Raskin, Daniel Hansen, Gaddum D. Reddy, Andrew Jea, Sandi Lam. Surgical management of lipomyelomeningocele in children: Challenges and considerations. 26-Apr-2017;8:63. Available from: http://surgicalneurologyint.com/surgicalint-articles/surgical-management-of-lipomyelomeningocele-in-children-challenges-and-considerations/
Keywords: Spinal lipoma, lipomyelomeningocele, spinal dysraphism, pediatric
ILLUSTRATIVE CASES
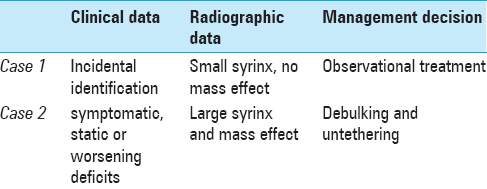
Case 1
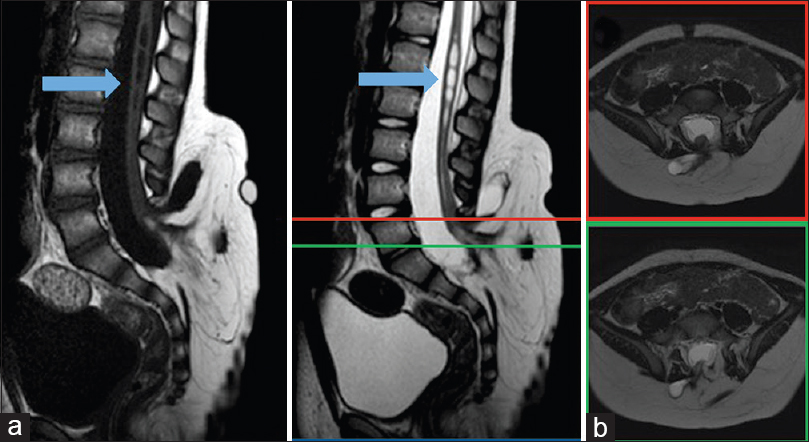
A 3-month-old boy was referred to neurosurgery when a subcutaneous bulge in the lower lumbar region was incidentally noted. Initial workup included an ultrasound of the region that was concerning for spinal dysraphism, including sacral agenesis and an associated intraspinal mass. These findings prompted a lumbosacral magnetic resonance image (MRI), which confirmed the diagnosis of lipomyelomeningocele. The patient was clinically asymptomatic, with normal strength in his lower extremities, no evidence of hydrocephalus, and normal bowel and bladder function. Because he was meeting developmental milestones, he was managed observantly with annual clinical exams, which remained normal. At 3 years of age, he underwent urodynamic studies, which were unremarkable, and he was able to successfully toilet train. At around this time, his parents reported transient morning stiffness in the back and lower legs, which would resolve by the afternoon. This prompted a repeat MRI [
Case 2
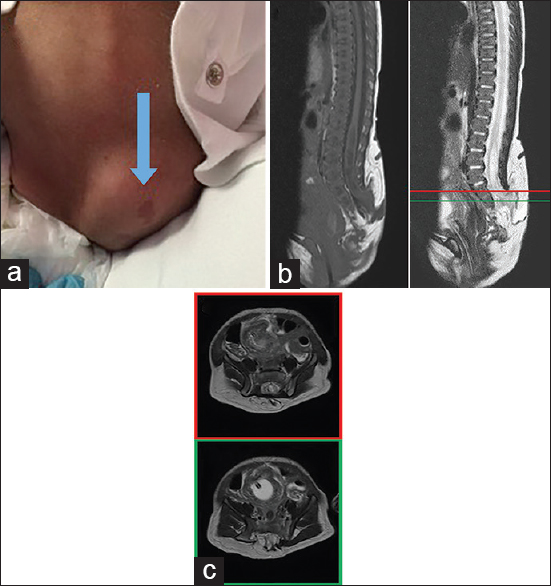
An 11-day-old girl with an uncomplicated birth was noted by her parents to have a lump on her lower back, prompting further workup by her pediatrician. A physical exam revealed a 4–5 cm soft, nontender mass in the lumbar spine with hyperpigmented changes [
INTRODUCTION
Lipomyelomeningocele (LMMC) is a closed neural tube defect in which neural elements are incorporated into a spinal lipoma. This is an uncommon defect, occurring in 3–6 patients per 100,000 live births.[
Embryology
Central nervous system development consists of primary and secondary neurulation. During primary neurulation, the notochord induces folding of the neural plate to form the neural tube, which extends in both the rostral and caudal directions. The ectoderm overlying the neural tube separates to ultimately form the skin dorsal to the spine, a process known as dysjunction. Mesoderm around the neural tube differentiates into the posterior vertebral elements, fat, and paraspinal musculature. In premature dysjunction, mesoderm can migrate into the neural tube before it is fully closed, disrupting the neurulation process. This mesoderm then differentiates into fat and forms a border between the neural placode and the now entrapped lipoma. As development continues, meninges form around the neural tube except at the placode-lipoma interface, leaving a dorsal diaschisis traversed by a lipoma. This often results in a distinct transition point between normal planes and anatomy, anteriorly, and the lipoma, posteriorly.[
In secondary neurulation, a caudal mass of mesenchymal mesoderm cavitates and fuses with the primary neural tube, forming the spine below S2. After fusion of the primary and secondary neural tubes, mesoderm can migrate caudally and interfere with secondary neurulation in a mechanism similar to the disruption of primary neurulation. Secondary neurulation differs phylogenetically and is incompletely understood. Humans lack mature tail structure and have less complexity of secondary neurulation comparatively. In chick embryos, dynamic histology describes a coalescing of radially oriented tubules around a central lumen, ultimately cavitating within the caudal cell mass and joining the primary neurulated structure. Prevailing theories involve morphogenetic determinants, with candidate genes including sonic hedgehog and Pax transcription factors.[
The morphology of spinal lipomas is thought to be determined by which of the two neurulation processes is affected. Regardless of the morphology, in LMMC the placode-lipoma junction lies outside the spinal canal with dorsal extension of the meninges through an accompanying bony defect, in contrast to residing inside the canal, as seen in a lipomyelocele.[
Classification
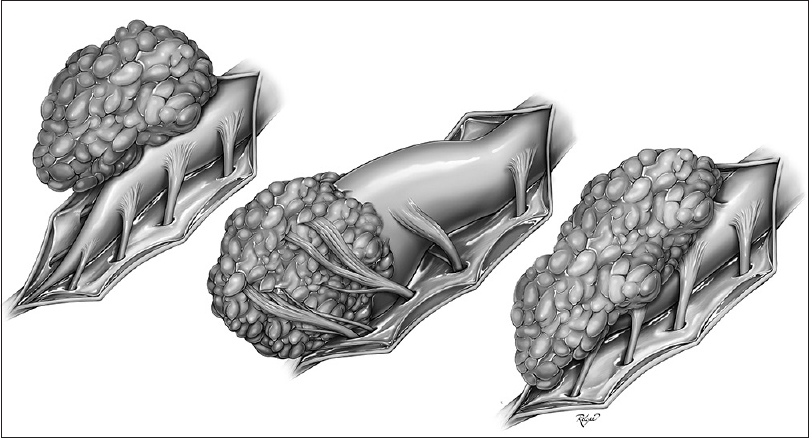
Traditionally, spinal lipomas have been classified into three groups based on the location of the neural placode–lipoma junction: Dorsal, caudal, and transitional, known as the Chapman classification[
Premature dysjunction is necessary for all types of spinal lipomas, except the chaotic type. Primary and secondary neurulation is disrupted in the dorsal and caudal types, respectively, whereas both are affected in the transitional type. The chaotic type is thought to involve only secondary neurulation, where mesenchymal cells may become mixed with the caudal stem cell mass.
Presentation
Spinal lipomas and LMMCs are frequently associated with cutaneous and musculoskeletal abnormalities in addition to sensorimotor deficits and urological dysfunction.[
At birth, neurological symptoms may be absent in nearly half of the cases.[
LMMC can be associated with additional pathologies, including Chiari malformation type 1 (13%), spina bifida (14.4%), split cord malformations (3.1%), associated dermal sinuses (3.1%), dermoid or epidermoid cysts (3.1%), diastematomyelia (3.1%), terminal hydromyelia (3.1%), anal stenosis (1.0%), and Down syndrome (1.0%).[
DIAGNOSTIC STUDIES
Ultrasound is an effective screening tool because it is low risk and widely available, however, it has limited use after the initial diagnosis or following surgical treatment and should not be relied upon as the sole preoperative assessment.[
MANAGEMENT
Historical studies have shown that surgical interventions may briefly stabilize or relieve neurological symptoms but ultimately fail to improve upon the natural history of LMMCs.[
Surgical intervention may provide temporary relief or lessening of symptoms by releasing tension on the spinal cord, however, there is a risk of retethering with subsequent return or progression of neurologic symptoms, with reported rates of 5–50%. In a study by Colak et al. of 94 patients who underwent initial repair of a LMMC, 20.2% required subsequent operations for symptomatic retethering, with an average follow-up of 52 months after surgery. Of these reoperated patients, 6.4% exhibited repetitive symptomatic tethering, which became more difficult to treat and with shorter times between return of symptoms. Colak concluded that even after an adequate initial operation, symptomatic retethering is a common problem and that no current duraplasty graft material entirely prevents this from occurring.[
A recent retrospective review attempted to identify radiological correlates predicting neurological decline. Over 16 years, a 24-patient population with LMMC that underwent an observational management strategy at a single institution was dichotomized into those experiencing early (less than 18 months) and late (18–30 months) neurological deterioration. Nine patients experiencing early deterioration were more likely to have large intradural lipomatous masses, which grew within the first year to exert regional mass effect on neural structures and were associated with a large expanded syrinx. Early decliners were more likely to present with motor deficits, whereas 15 patients experiencing late neurological decline presented with mixed urologic and motor deficits.[
Several factors are thought to affect the treatment outcome of LMMCs. Age, gender, morphology, the presence and severity of neurological symptoms, and absence or presence of an associated spinal cord syrinx are all taken into consideration. Of these, morphology is considered the most crucial factor affecting outcome. For example, transitional lipomas appear to have a higher rate of retethering after surgery than dorsal and caudal types.[
The traditional surgical technique described in historical studies involves partial resection of the lipoma to avoid injury to the neural placode, followed by untethering of the cord, then apposition of the edges of the placode, and finally duraplasty.[
A radically different approach to the treatment of tethered cord comes in the form of vertebral column shortening, which offers an alternative method for relieving tension on the spinal cord without risking injury to the neural placode and possibly stabilizing or improving neurological outcome.[
Regardless of the type of surgical intervention, the use of operative microscope is recommended, and intraoperative neurophysiological monitoring should be performed. There are a number of various neurophysiologic measures that can be used, and institutional practice may dictate what is available. Whatever monitoring type is chosen, the goal remains to avoid unintended injury to intact nervous structures, which may be hidden by or attached to the LMMC.[
As mentioned earlier, management of symptomatic LMMCs may not always entail surgical untethering. Complex variants of the transitional or chaotic spinal lipomas associated with isolated urological symptoms or orthopedic deformities may be observed because these abnormalities are less likely to improve with surgical intervention. They also carry a higher risk of unsuccessful untethering and incomplete lipoma resection, which may lead to an increased risk of neurological deterioration after surgery.
After careful evaluation, “simple” LMMCs with symptoms or any LMMC with an associated sensorimotor deficit should be considered for possible surgical intervention. Caudal and dorsal spinal lipomas are typically more amenable to surgical treatment than the rest. Immediate postoperative complication rates range from 10–30% and include infection, CSF leak, or neurological deterioration.[
LMMCs associated with embryomorphic malformations of other systems may represent the more severe end of the spectrum of congenital defects. For example, OEIS (omphalocele, exstrophy, imperforate anus, spinal defects) and VATER (vertebral defects, anal atresia, tracheoesophageal fistula, renal abnormalities) represent associated multisystem abnormalities, which complicate management and are beyond the scope of this review.
CASES IN CONTEXT
CONCLUSION
LMMC management remains a challenge. The selected cases demonstrate important factors integrated within a clinical decision rule. Although there is no high-quality clinical outcome data to provide guidance regarding the treatment options for LMMCs, conservative management of asymptomatic patients is appropriate. Clearly progressive symptomatic patients should be considered for surgical untethering with the goal of managing symptoms, with the patient and family prepared for an iterative process. Patients with static neurological deficits should be managed observationally. Prophylactic surgery may, theoretically, prevent the onset of neurological deterioration or stabilize and reverse early-onset symptoms at diagnosis, especially in infants with large intradural lipoma and associated syrinx, which compress neural structures, however, this has not been shown to offer immunity against further deterioration. When surgical management is elected, experts advocate aggressive resection of the lipoma, along with reconstruction of the placode and large expansile duraplasty, but the literature shows this is technically difficult and may not greatly improve upon the natural history of LMMCs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Arai H, Sato K, Okuda O, Miyajima M, Hishii M, Nakanishi H. Surgical experience of 120 patients with lumbosacral lipomas. Acta Neurochir. 2001. 143: 857-64
2. Atala A, Bauer SB, Dyro FM, Shefner J, Shillito J, Sathi S. Bladder functional changes resulting from lipomyelomeningocele repair. J Urol. 1992. 148: 592-4
3. Blount JP, Elton S. Spinal lipomas. Neurosurg Focus. 2001. 10: e3-
4. Byrne RW, Hayes EA, George TM, McLone DG. Operative resection of 100 spinal lipomas in infants less than 1 year of age. Pediatr Neurosurg. 1995. 23: 182-6
5. Chapman PH. Congenital intraspinal lipomas: Anatomic considerations and surgical treatment. Childs Brain. 1982. 9: 37-47
6. Cochrane DD. Cord untethering for lipomyelomeningocele: Expectation after surgery. Neurosurg Focus. 2007. 23: E9-
7. Colak A, Pollack IF, Albright AL. Recurrent tethering: A common long-term problem after lipomyelomeningocele repair. Pediatr Neurosurg. 1998. 29: 184-90
8. Copp AJ, Stanier P, Greene ND. Neural tube defects: Recent advances, unsolved questions, and controversies. Lancet Neurol. 2013. 12: 799-810
9. Cornette L, Verpoorten C, Lagae L, Plets C, Van Calenbergh F, Casaer P. Closed spinal dysraphism: A review on diagnosis and treatment in infancy. Eur J Paediatr Neurol. 1998. 2: 179-85
10. Detrait ER, George TM, Etchevers HC, Gilbert JR, Vekemans M, Speer MC. Human neural tube defects: Developmental biology, epidemiology, and genetics. Neurotoxicol Teratol. 2005. 27: 515-24
11. Dorward NL, Scatliff JH, Hayward RD. Congenital lumbosacral lipomas: Pitfalls in analysing the results of prophylactic surgery. Childs Nerv Syst. 2002. 18: 326-32
12. Drolet B. Birthmarks to worry about. Cutaneous markers of dysraphism. Dermatol Clin. 1998. 16: 447-53
13. Finn MA, Walker ML. Spinal lipomas: Clinical spectrum, embryology, and treatment. Neurosurg Focus. 2007. 23: E10-
14. Forrester MB, Merz RD. Descriptive epidemiology of lipomyelomeningocele, Hawaii, 1986-2001. Birth Defects Res A Clin Mol Teratol. 2004. 70: 953-6
15. Guggisberg D, Hadj-Rabia S, Viney C, Bodemer C, Brunelle F, Zerah M. Skin markers of occult spinal dysraphism in children: A review of 54 cases. Arch Dermatol. 2004. 140: 1109-15
16. Hertzler DA, DePowell JJ, Stevenson CB, Mangano FT. Tethered cord syndrome: A review of the literature from embryology to adult presentation. Neurosurg Focus. 2010. 29: E1-
17. Hoffman HJ, Hendrick EB, Humphreys RP. The tethered spinal cord: Its protean manifestations, diagnosis and surgical correction. Childs Brain. 1976. 2: 145-55
18. Hoffman HJ, Taecholarn C, Hendrick EB, Humphreys RP. Management of lipomyelomeningoceles. Experience at the Hospital for Sick Children, Toronto. J Neurosurg. 1985. 62: 1-8
19. Hoving EW, Haitsma E, Oude Ophuis CM, Journee HL. The value of intraoperative neurophysiological monitoring in tethered cord surgery. Childs Nerv Syst. 2011. 27: 1445-52
20. Hsieh PC, Stapleton CJ, Moldavskiy P, Koski TR, Ondra SL, Gokaslan ZL. Posterior vertebral column subtraction osteotomy for the treatment of tethered cord syndrome: Review of the literature and clinical outcomes of all cases reported to date. Neurosurg Focus. 2010. 29: E6-
21. Huang SL, Shi W, Zhang LG. Surgical treatment for lipomyelomeningocele in children. World J Pediatr. 2010. 6: 361-5
22. . International Society of Ultrasound in O, Gynecology Education C. Sonographic examination of the fetal central nervous system: Guidelines for performing the ‘basic examination’ and the ‘fetal neurosonogram’. Ultrasound Obstet Gynecol. 2007. 29: 109-16
23. Kanev PM, Lemire RJ, Loeser JD, Berger MS. Management and long-term follow-up review of children with lipomyelomeningocele, 1952-1987. J Neurosurg. 1990. 73: 48-52
24. Kannu P, Furneaux C, Aftimos S. Familial lipomyelomeningocele: A further report. Am J Med Genet A. 2005. 132A: 90-2
25. Khealani B, Husain AM. Neurophysiologic intraoperative monitoring during surgery for tethered cord syndrome. J Clin Neurophysiol. 2009. 26: 76-81
26. Kokubun S, Ozawa H, Aizawa T, Ly NM, Tanaka Y. Spine-shortening osteotomy for patients with tethered cord syndrome caused by lipomyelomeningocele. J Neurosurg Spine. 2011. 15: 21-7
27. Kothbauer KF, Novak K. Intraoperative monitoring for tethered cord surgery: An update. Neurosurg Focus. 2004. 16: E8-
28. Kulkarni AV, Pierre-Kahn A, Zerah M. Conservative management of asymptomatic spinal lipomas of the conus. Neurosurgery. 2004. 54: 868-73
29. Muthukumar N. Congenital spinal lipomatous malformations: Part I--Classification. Acta Neurochir. 2009. 151: 179-88
30. Pang D, Zovickian J, Wong ST, Hou YJ, Moes GS. Surgical treatment of complex spinal cord lipomas. Childs Nerv Syst. 2013. 29: 1485-13
31. Pierre-Kahn A, Zerah M, Renier D, Cinalli G, Sainte-Rose C, Lellouch-Tubiana A. Congenital lumbosacral lipomas. Childs Nerv Syst. 1997. 13: 298-334
32. Tu A, Hengel AR, Cochrane DD. Radiographic predictors of deterioration in patients with lumbosacral lipomas. J Neurosurg Pediatr. 2016. 18: 171-6
33. Tubbs RS, Bui CJ, Rice WC, Loukas M, Naftel RP, Holcombe MP. Critical analysis of the Chiari malformation Type I found in children with lipomyelomeningocele. J Neurosurg. 2007. 106: 196-200
34. Wu HY, Kogan BA, Baskin LS, Edwards MS. Long-term benefits of early neurosurgery for lipomyelomeningocele. J Urol. 1998. 160: 511-4
35. Wykes V, Desai D, Thompson DN. Asymptomatic lumbosacral lipomas--A natural history study. Childs Nerv Syst. 2012. 28: 1731-9