- Department of Neurosurgery, All India Institute of Medical Sciences, Patna, Bihar, India.
Correspondence Address:
Saraj Kumar Singh, Department of Neurosurgery, All India Institute of Medical Sciences, Patna, Bihar, India.
DOI:10.25259/SNI_374_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Anand Kumar Das, Suraj Kant Mani, Saraj Kumar Singh. Surgical management of post-COVID invasive rhino-orbito-cerebral mucormycosis and its outcomes: Role of neurosurgeons in a tertiary care center. 05-Aug-2022;13:335
How to cite this URL: Anand Kumar Das, Suraj Kant Mani, Saraj Kumar Singh. Surgical management of post-COVID invasive rhino-orbito-cerebral mucormycosis and its outcomes: Role of neurosurgeons in a tertiary care center. 05-Aug-2022;13:335. Available from: https://surgicalneurologyint.com/surgicalint-articles/11772/
Abstract
Background: Mucormycosis, which was previously considered to be rare, has emerged with a new challenge in patients infected with or recovering from COVID-19. Immunocompromised patients are particularly prone to developing this disease. The most common form of presentation is rhino-orbito-cerebral mucormycosis (ROCM). We present various neurosurgical approaches to an entire spectrum of its clinical manifestations.
Methods: This is a retrospective study of patients who were admitted to the neurosurgery department with ROCM and a history of COVID-19 infection between November 1, 2020, and September 1, 2021. All cases of ROCM underwent contrast-enhanced computed tomography/magnetic resonance imaging of the brain, paranasal sinuses, and orbit. A tissue biopsy was sent for histopathological analysis. All confirmed cases received liposomal amphotericin B and surgical treatment was immediately undertaken.
Results: Out of 200 patients with ROCM, 40 patients presented with neurological manifestations. Seven out of 40 patients had focal lesions in the brain and skull bone that needed neurosurgical intervention along with sinus debridement and antifungal treatment. These seven patients presented with different clinical manifestations: large-vessel stroke (one), medium-vessel stroke (one), frontal lobe abscess (one), frontal bone osteomyelitis (two), isolated central nervous system involvement (one), and mucor mimicking trigeminal schwannoma (one). The surgical intervention included decompressive craniectomy, frontal craniotomy, subtemporal craniotomy, and a minimally invasive supraorbital keyhole approach.
Conclusion: In high-risk patients, a high level of clinical suspicion combined with appropriate investigations should be performed as soon as possible. Symptoms and early warning signs should not be overlooked, as treatment delays can be fatal. A minimally invasive surgical approach is possible in view of decreasing the morbidity of large craniotomy.
Keywords: Abscess, COVID-19, Mucormycosis, Osteomyelitis, Stroke
INTRODUCTION
Mucormycosis is an invasive fungal infection caused by Zygomycete fungi of the family Mucoraceae.[
ROCM management must be a multidisciplinary effort involving an otorhinolaryngologist at the center, as well as an ophthalmologist, neurologist/neurosurgeon, anesthetists, plastic surgeons, and rehabilitation professionals.[
MATERIALS AND METHODS
We performed a retrospective study of patients who were admitted to our neurosurgery department with ROCM and current or past history of COVID-19 infection between November 1, 2020, and September 1, 2021. We included only those patients who required neurosurgical intervention. All participants who took part in the study signed a written and well-informed consent form. The study was carried out in accordance with the Declaration of Helsinki. The demographic profile, clinical presentation, examination and imaging findings, and laboratory findings were retrieved from records. All suspected cases of ROCM had contrast-enhanced computed tomography (CECT) of the paranasal sinus, orbit, and brain. For patients with an intracranial extension on CECT, contrast-enhanced magnetic resonance imaging (CEMRI) of the brain, orbit, and paranasal sinus was obtained. A tissue biopsy was taken from the nasal cavity and sent for histopathological analysis and culture. All patients received liposomal amphotericin B after confirmation of histopathology and surgical treatment was undertaken immediately.
CASE HISTORY
Case 1 (Large-vessel stroke)
A 66-year-old nonsmoker and nonalcoholic male with a medical history of hypertension and COVID-19 infection presented to hospital emergency with altered mental status. He had a headache, swelling over the right eye and face with the right side nasal blockage, and nasal bleeding for 7 days. He had a high-grade fever for the past 5 days. There was a history of COVID-19-associated pneumonia 1 month back, for which he was admitted to ICU and was treated conservatively with high-flow oxygen, antibiotics, steroids, and low-molecular-weight heparin.
On the initial assessment, the patient was drowsy. The temperature was 102°F, pulse – 64/min with a blood pressure of 150/96 mm Hg. He was tachypneic (30/min) with oxygen saturation of 88%. Random blood sugar was 457 mg/dl. Initial Glasgow Coma Scale (GCS) was 8 (E1 V2 M5) with the left side hemiplegia, upper motor neuron Type 7 nerve palsy, and right-sided ptosis. There were no signs of meningeal irritation. There was blackish discoloration around both eyes, the right side conjunctiva was diffusely chemosis. The right side pupil was dilated (5–6 mm) and not reacting to light and the left was of normal size but sluggishly reacting to light. The patient was sedated and intubated immediately. Mechanical ventilation was initiated along with anti-edema measures (mannitol + hypertonic saline). The patient was started on intravenous fluids and insulin to optimize his hyperglycemic state. Empirical antibiotics and antiepileptics were also started. His white blood cells were significantly elevated with markedly increased neutrophils. His renal function and serum electrolytes were normal.
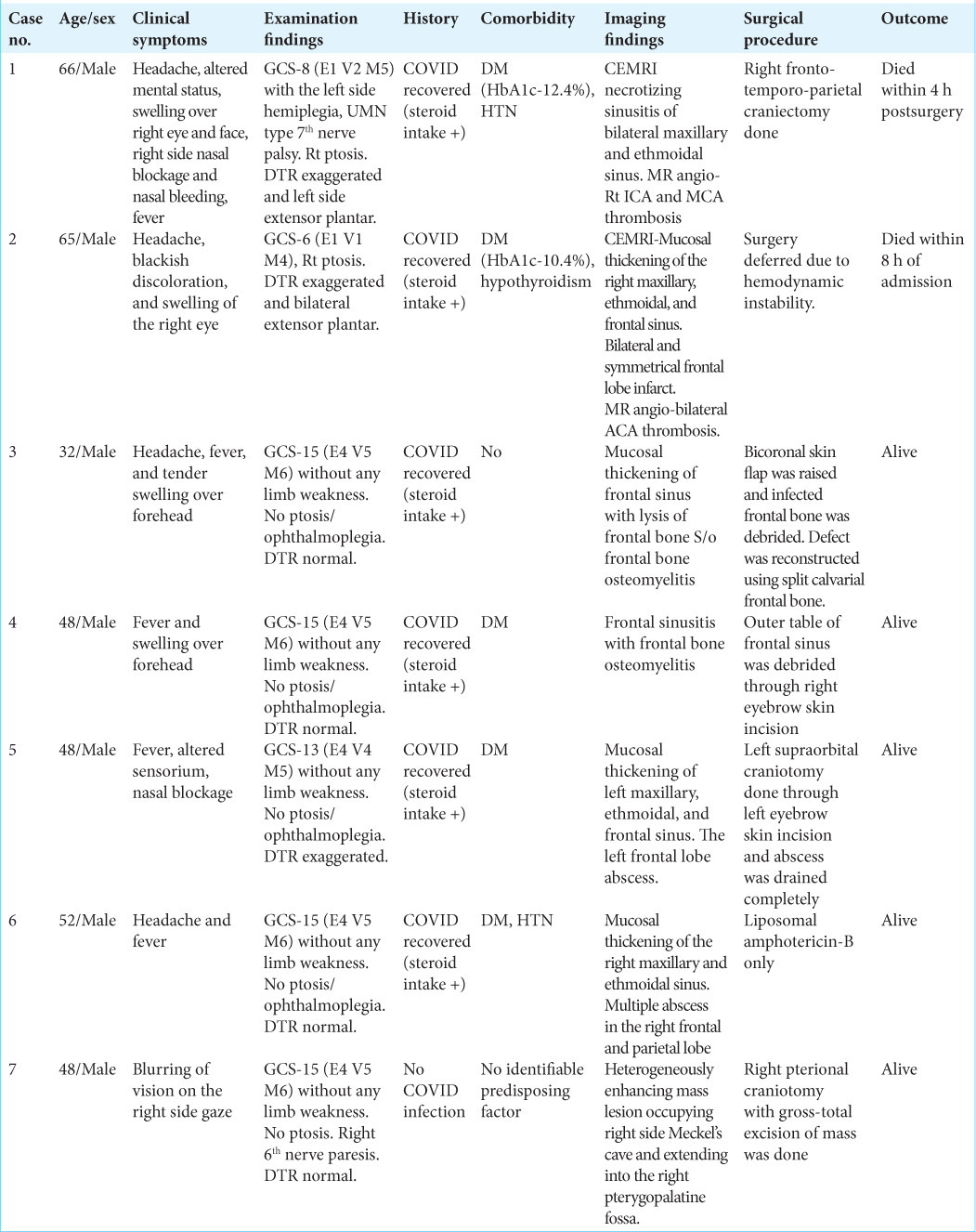
Contrast MRI brain with angiography showed necrotizing sinusitis of bilateral maxillary and ethmoidal sinus with extensive thickening of bilateral buccinator space, right pterygopalatine fossa, and right premaxillary region. There was an absence of flow voids of the right internal carotid artery (ICA) and right middle cerebral artery (MCA) suggestive of the right ICA and MCA thrombosis (long segment). The contralateral anterior cerebral artery (ACA) was filling the anterior communicating artery and ipsilateral ACA. There was diffusion restriction of the right cerebral hemisphere with significant mass effect and midline shift [
Figure 1:
(a) Axial high-resolution computed tomography chest image showing bilateral patchy areas of ground-glass opacities with the left lung lower lobe consolidation (red star) (1 month before presentation), (b) coronal postcontrast T1-weighted fat saturation (T1W-FS) image showing heterogeneous enhancing soft tissue in the left maxillary and ethmoid sinuses (yellow arrow) with nonenhancing right turbinates and maxillary sinuses (yellow star), (c) coronal postcontrast T1W-FS image showing thrombosed right cavernous sinus and absent flow void of the right internal carotid artery (ICA) (yellow arrow), and (d) coronal T2-weighted fat saturation image showing the heterogeneous hyperintense appearing of the right cavernous sinus with ICA (blue arrowhead) and direction of spread (shaft of arrow). Opposite ICA is intact (red arrow), (e) three-dimensional time-of-flight MR angiography, maximum intensity projection, showing nonvisualization of the right internal carotid and middle cerebral arteries (dashed oval), (f) axial diffusion-weighted image showing restricted diffusion in large area of the right cerebral hemisphere, (g) KOH stained magnified image (×40) showing sporangium containing sporangiospores (thick blue arrow) with sporangiophore and nonseptate hyphae, and (h) lactophenol cotton blue stained magnified image (×40) showing sporangium (yellow arrowhead) with sporangiophore (red arrowhead).
Case 2 (Medium-vessel stroke)
A 65-year-old male presented in emergency with an altered sensorium with the complaints of frontal headache, blackish discoloration, and swelling of the right eye for the past 3 days. He had a history of diabetes and hypothyroidism for which he was taking medication. He tested positive for COVID-19 and took home-based management for 7 days before presenting to us. On initial assessment, the patient was unconscious, tachypneic (40/min) with oxygen saturation of 80% on a high-flow oxygen mask. His temperature was 100°F, pulse – 94/min, and had a blood pressure of 160/100 mmHg. His blood investigation showed a random blood sugar of 386 mg/dl. His Glasgow Coma Score was 6 (E1 V1 M4). There were no signs of meningeal irritation. His right-sided conjunctiva was diffusely chemosis. The right pupil was dilated, sluggishly reacting to light, and left-sided pupil was of normal size reacting to light. The right side ptosis was present.
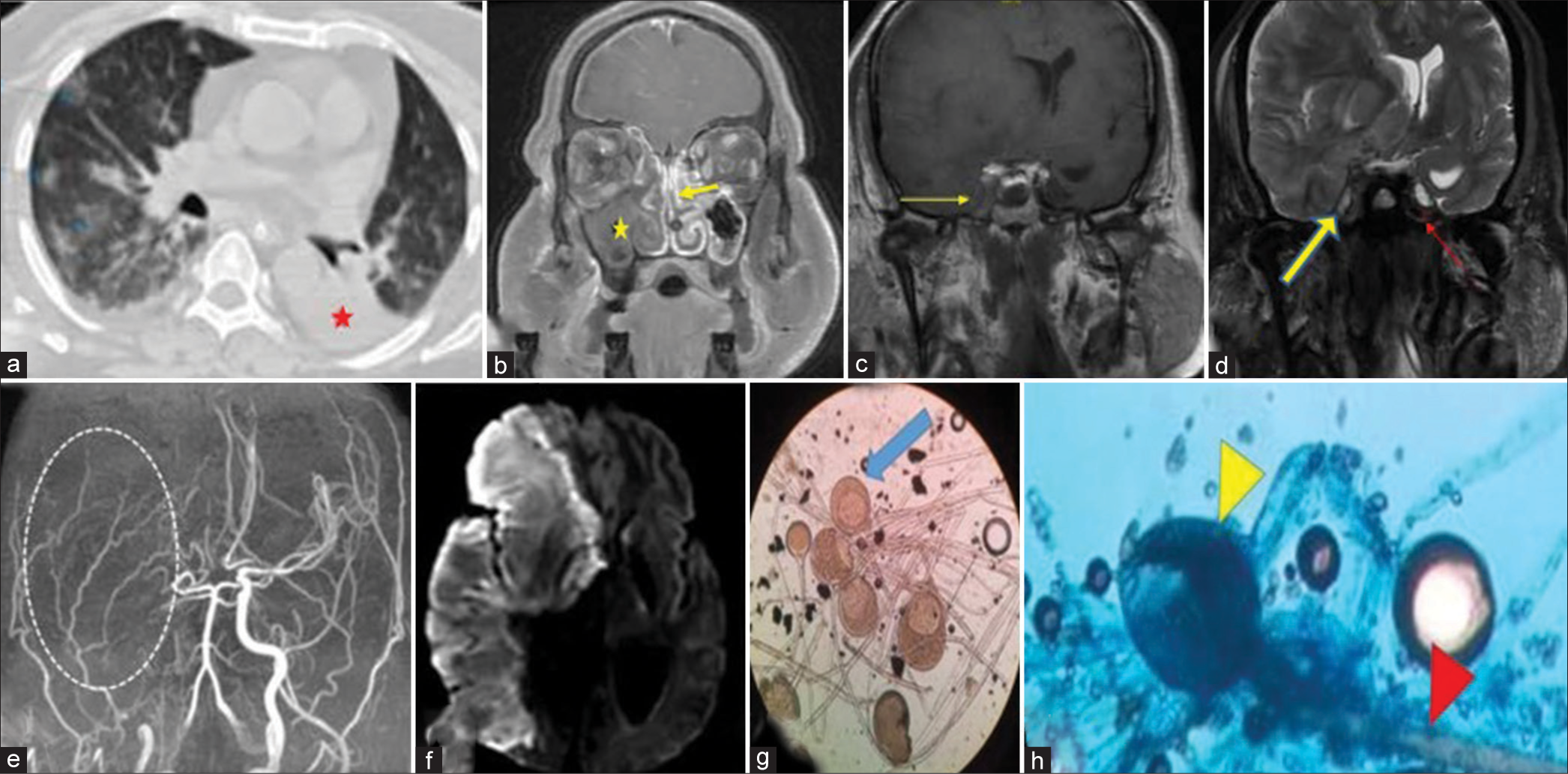
MRI brain with paranasal sinus showed mucosal thickening of the right maxillary, ethmoidal, and frontal sinus. There was a lysis of planum ethmoidale and cribriform plate. Bilateral and symmetrical frontal lobe infarcts with an involvement of genu and anterior body of corpus callosum. The involved area showed diffusion restriction along with the disappearance of gray-white matter interface with significant mass effect suggestive of anterior cerebral artery territory stroke [
Figure 2:
(a) Axial high-resolution computed tomography chest image showing bilateral patchy areas of ground-glass opacities (green arrowheads), (b) sagittal T2W image showing hyperintense region of the frontal lobe (yellow star), note direct contiguity of abnormal high T2 signal between the frontal lobe and similar lesion in the ethmoid sinus (yellow arrow), (c) three-dimensional time-of-flight MR angiography, maximum intensity projection image showing lack of visualization of both anterior cerebral arteries (white oval region points to the normal expected locations), and (d) axial diffusion-weighted image showing restriction in both frontal lobes.
With the above features in a post-COVID patient, a presumptive diagnosis of invasive fungal infection was made. Tissue scrapings from the nose showed growth of fungal elements – suggestive of mucormycosis. The patient was started on liposomal amphotericin B (10 mg/kg). Urgent surgical decompression (craniectomy) and sinus debridement were planned. However, within 3 h of admission, the patient deteriorated to GCS-3 (E1M1V1) with fixed dilated pupils on both sides. His brain stem reflexes were absent. Due to hemodynamic instability, the surgical plan was deferred. The patient immediately started vasopressors but could not sustain and succumbed within 8 h of admission despite all measures.
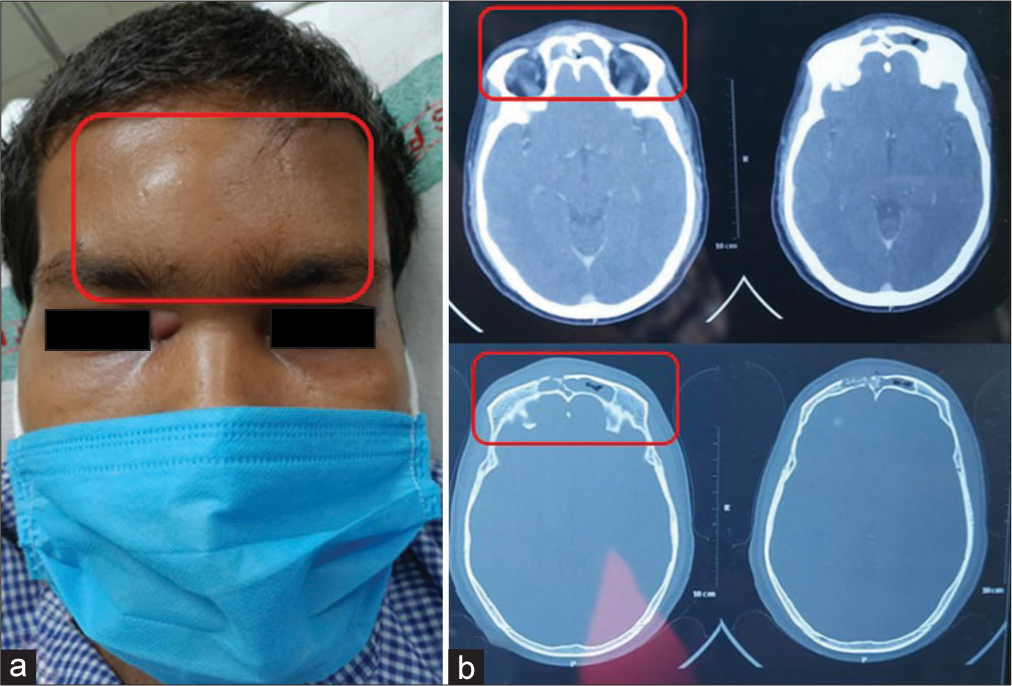
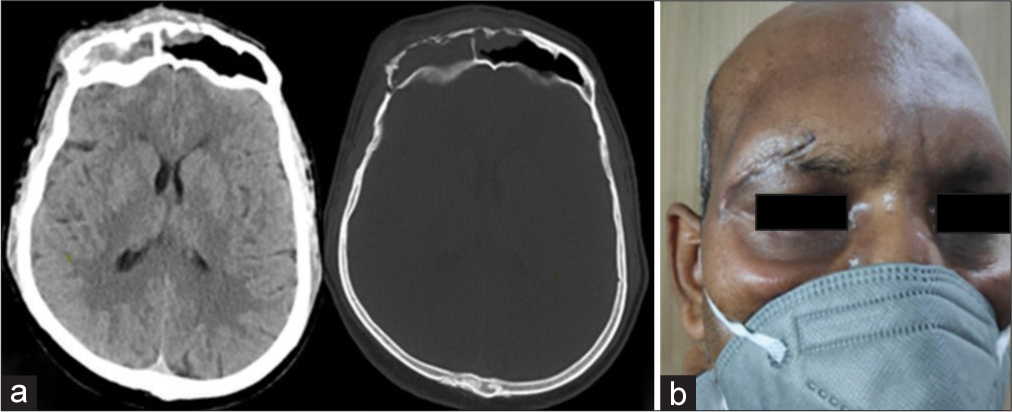
Case 3 (Frontal bone osteomyelitis)
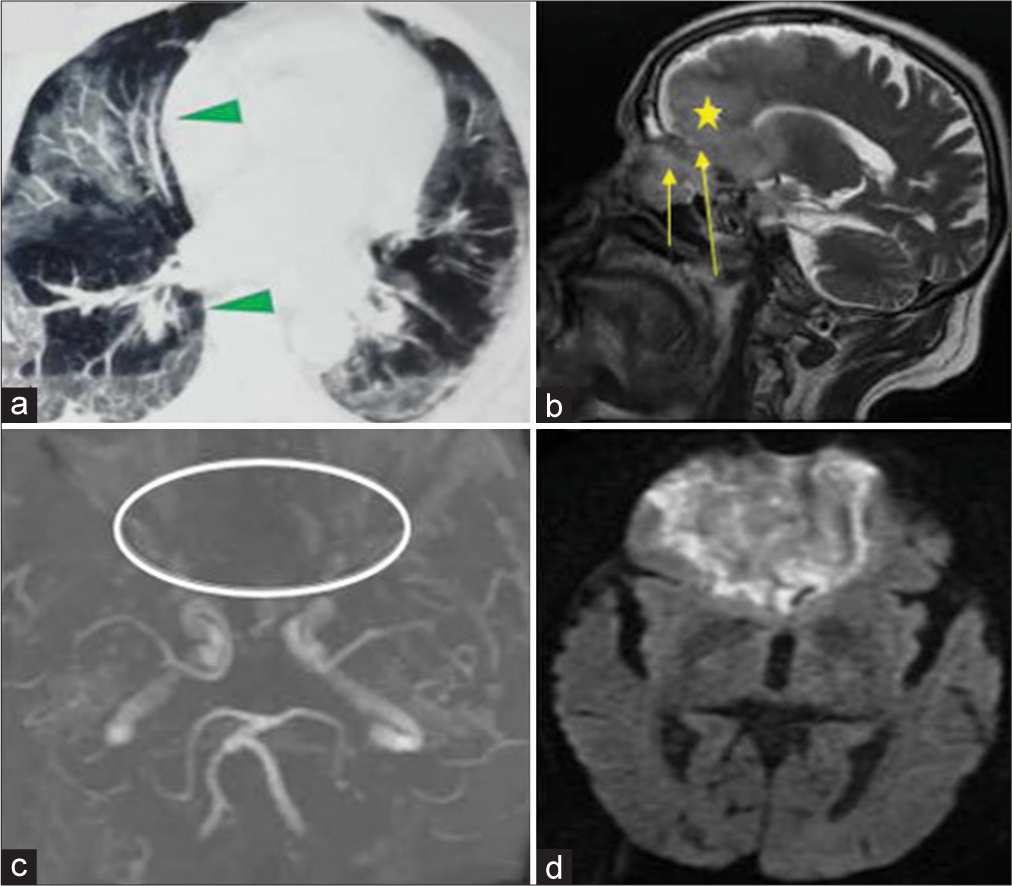
A 32-year-old male presented with the chief complaints of headache, fever, and tender swelling over the forehead for the past 10 days [
Case 4 (Frontal bone osteomyelitis)
A 48-year-old male presented with fever and swelling over the forehead for the past 5 days. There was no h/o headache, seizure, or nasal blockage. The patient was conscious and oriented. He had a history of DM for which he was taking insulin. He was also admitted for COVID-19-associated pneumonia 1 month back for which he was given antibiotics, anticoagulant, and steroids. Neurological examination was completely normal. Noncontrast computed tomography brain showed frontal sinusitis with osteomyelitis [
Case 5 (Left frontal lobe abscess)
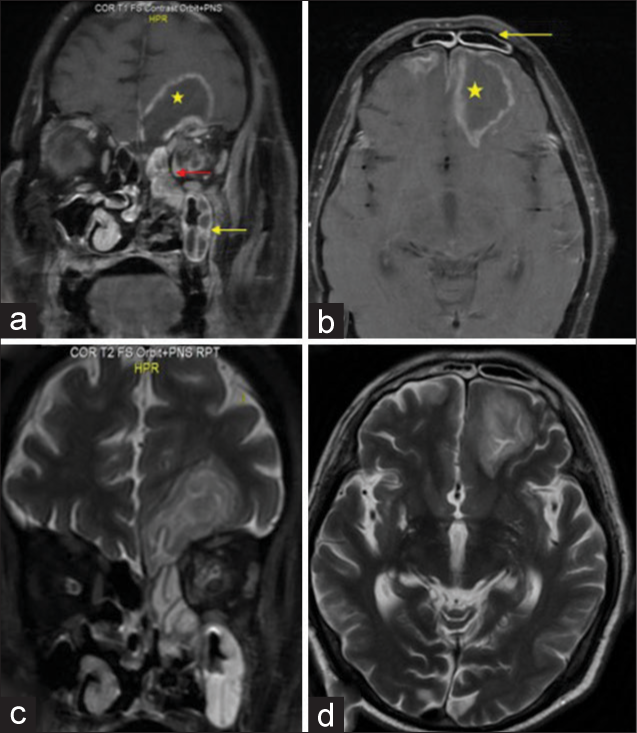
A 48-year-old male presented with the chief complaints of fever and nasal blockage for 5 days and altered sensorium for the past 2 days. The patient was a known diabetic for the past 3 years. One week back, only he was discharged after getting treatment for COVID infection. He was on oral antibiotics, anticoagulants, and a tapering dose of steroids. On examination, his GCS was 13 (E4 V4 M5) with no limb weakness. No ptosis was present. Ophthalmoplegia could not be assessed due to the confused status of the patient. Deep tendon reflexes were exaggerated. MRI brain and paranasal sinus were advised which revealed mucosal thickening of the left maxillary, ethmoid, and frontal sinus with the left frontal abscess (6 × 5.2 × 3.3 cm) [
Figure 5:
(a) Coronal postcontrast T1-weighted fat saturation (T1W-FS) image of brain and paranasal sinus showing heterogeneous enhancing soft tissue in the left maxillary (yellow arrow) and ethmoid sinus (red arrow) with contiguous extension to rim-enhancing lesion in the left frontal lobe (yellow star), (b) axial postcontrast T1W-FS image showing mucosal enhancement of frontal sinus (yellow arrow) and the left frontal abscess (yellow star), (c) coronal T2-weighted image showing hyperintense maxillary and ethmoid sinus with hyperintense lesion in the left frontal lobe, and (d) axial T2W MRI brain showing hyperintense left frontal lesion.
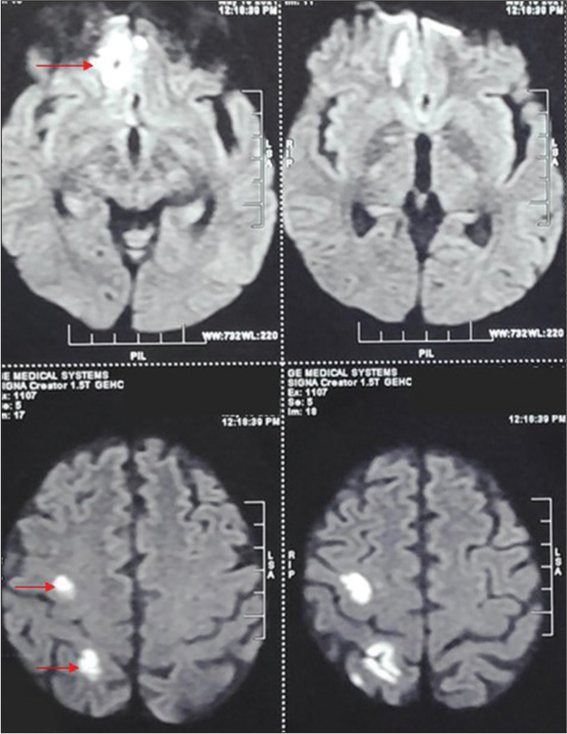
Case 6 (Isolated CNS involvement)
A 52-year-old male, known diabetic (uncontrolled on insulin), presented with the complaints of headache and fever for the past 10 days. There was no history of vomiting, seizure, decreased vision, or nasal blockage. He was on antihypertensive medications for the past 5 years. The patient was discharged 1 month back after 15 days of hospital stay for COVID-19-associated pneumonia. He received antibiotics, anticoagulants, and steroids and was on a noninvasive high-flow oxygen mask. On presentation, he was conscious with GCS – 15/15. There were no neck rigidity, limb weakness, ptosis, or ophthalmoplegia. CEMRI brain and paranasal sinus suggested multiple abscesses over the right frontoparietal region with mucosal thickening of the right maxillary and ethmoid sinus. These lesions showed diffuse restriction [
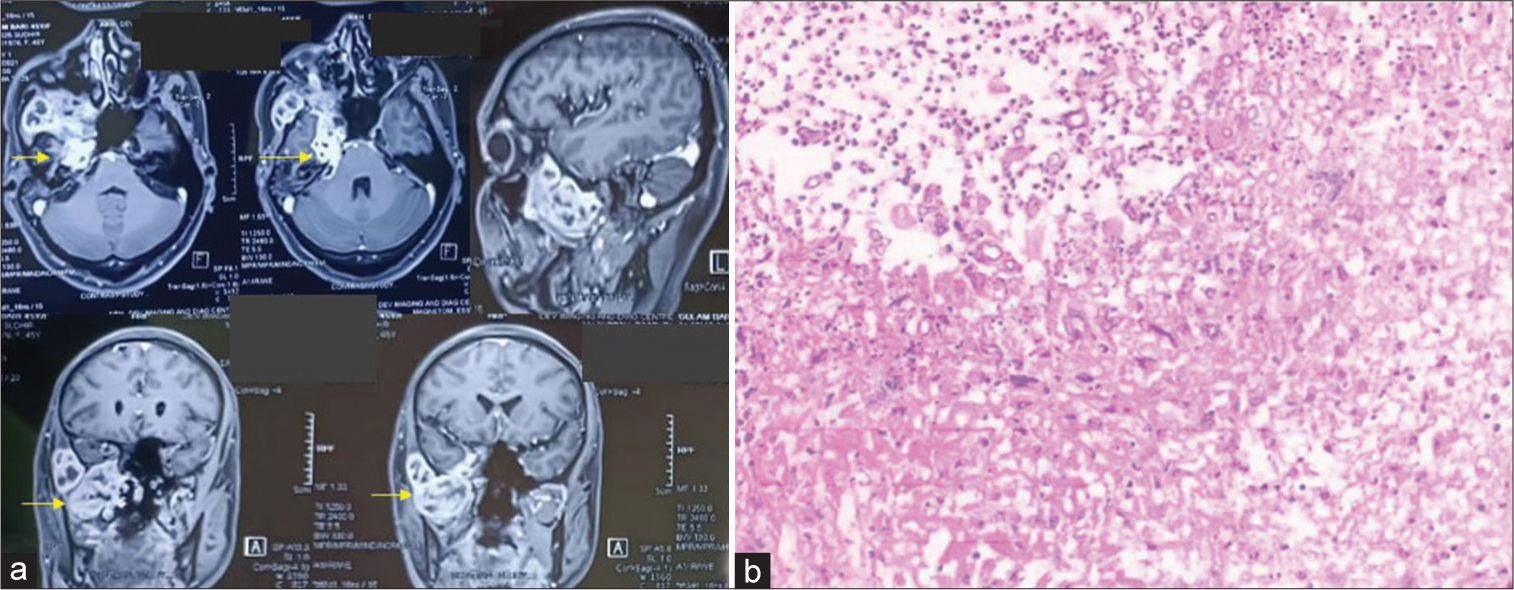
Case 7 (ROCM mimicking trigeminal schwannoma)
A 48-year-old nondiabetic, nonhypertensive, and nonsmoker male presented to us with the only complaint of blurring of vision in the right gaze. There was no history of headache, vomiting, seizure, fever, or nasal blockage. There was no history of COVID-19 infection or any symptoms suggestive of it. On examination, GCS was 15/15 with no limb weakness. Ptosis was absent. The right side sixth nerve paresis was present with restricted lateral movement of the right eyeball. All other movements of the eyeball were normal. Deep tendon reflexes were normal. CEMRI brain was done which suggested a heterogeneously but avidly enhancing mass lesion occupying the right-sided Meckel’s cave and extending into the right pterygopalatine fossa, features suggestive of trigeminal schwannoma. No osteolytic features were present [
RESULTS
In our institution, we witnessed more than 200 patients of ROCM during the first and second waves of the COVID-19 pandemic. Out of these 200, 160 patients presented with blackish discharge from the nasal cavity, paranasal sinus mucosal thickening, and pale/blackish discoloration of the nasal mucosa and hard palate. These patients underwent sinus debridement with antifungal medications in the otolaryngology department. Forty patients presented with one or more neurological symptoms such as headache, facial pain, numbness, loss of vision, ptosis, and ophthalmoplegia suggestive of cavernous sinus thrombosis. Twenty-five out of 40 patients received antifungal and anticoagulant (injection Enoxaparin) along with sinus debridement from a combined effort of the medicine and otolaryngology department. Eight patients denied surgical debridement and were lost to follow-up. Seven patients had focal lesions in the brain and skull bone that needed neurosurgical intervention along with sinus debridement and antifungal treatment.
DISCUSSION
ROCM is an infrequent fatal invasive fungal infection that usually manifests in immunocompromised individuals.[
Here, we have briefed these seven cases to discuss the role of neurosurgeons and various approaches that can be offered to limit the comorbidity associated with surgery in the best possible way, as these patients have poor healing due to immunocompromised status. India bears a huge burden of DM among adults aged 20–79 years and it is the most common predisposing factor for mucormycosis.[
Angioinvasion with hematogenous spread is a characteristic feature of mucormycosis. Fungal hyphae proliferate along the internal elastic lamina, causing hyperplasia of intima and thrombosis, ultimately resulting in occlusion of vessels. COVID-19 infection exhibits a similar feature of thrombotic microangiopathy (due to upregulation of the prothrombotic CX3CL1 marker), which may have a synergic effect with mucormycosis, responsible for the fulminant spread of infection.[
The nasal cavity gets first affected following the inhalation of spores and results in discoloration and necrosis of the turbinates. The infection further extends to the paranasal sinuses and orbit causing cellulitis, ophthalmoplegia, and loss of vision and can also spread to the cavernous sinus with intracranial involvement.[
The intracranial spread occurs through vessels of the orbital apex (ophthalmic artery) or cribriform plate (ethmoidal artery) without causing any bone or dura involvement.[
Another course of invasion includes a perineural extension which is a very rare complication reported in post-COVID ROCM patients.[
When mucor infection presents with intracranial extension, the mortality rate escalates to be in the range of 50–80%. The survival rate depends on several factors such as early diagnosis, immediate control of hyperglycemia, removal of precipitating factors such as steroids, surgical debridement of the necrotic tissue, and antifungal therapy.[
In a developing country like India, where diabetes is more prevalent, COVID-19-infected diabetic patients should be closely monitored for the danger signs and symptoms of ROCM. Patients should be kept informed of the warning symptoms such as headache, facial numbness and tingling, and blurred vision, which should result in early detection of the disease.[
CONCLUSION
In high-risk patients, a high level of clinical suspicion combined with appropriate investigations should be performed as soon as possible. Symptoms and early warning signs of intracranial involvement should not be overlooked, as treatment delays can be fatal. A minimally invasive surgical approach is possible in view of decreasing the morbidity of large craniotomy.
Authors’ contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by all authors. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
I acknowledge all our professors and consultants in the department of neurosurgery for their guidance and assistance.
References
1. Agarwal V, Gupta A, Singh V, Jajodia N, Popli H, Akilan R. Association of COVID-19 with rhino-cerebral mucormycosis: An observational study. J Maxillofac Oral Surg. 2021. 11: 1-5
2. Al-Tawfiq JA, Alhumaid S, Alshukairi AN, Temsah MH, Barry M, Al Mutair A. COVID-19 and mucormycosis superinfection: The perfect storm. Infection. 2021. 49: 833-53
3. Balai E, Mummadi S, Jolly K, Darr A, Aldeerawi H. Rhinocerebral mucormycosis: A ten-year single centre case series. Cureus. 2020. 12: e11776
4. Cano P, Horseman MA, Surani S. Rhinocerebral mucormycosis complicated by bacterial brain abscess. Am J Med Sci. 2010. 340: 507-10
5. Dave TV, Gopinathan Nair A, Hegde R, Vithalani N, Desai S, Adulkar N. Clinical presentations, management and outcomes of rhino-orbital-cerebral mucormycosis (ROCM) Following COVID-19: A multi-centric study. Ophthalmic Plast Reconstr Surg. 2021. 37: 488-95
6. Dilek A, Ozaras R, Ozkaya S, Sunbul M, Sen EI, Leblebicioglu H. COVID-19-associated mucormycosis: Case report and systematic review. Travel Med Infect Dis. 2021. 44: 102148
7. Galletta K, Alafaci C, D’Alcontres FS, Maria ME, Cavallaro M, Ricciardello G. Imaging features of perineural and perivascular spread in rapidly progressive rhino-orbital-cerebral mucormycosis: A case report and brief review of the literature. Surg Neurol Int. 2021. 12: 245
8. Goyal A, Soni K, Sharma V, Choudhury B, Verma A, Kumar D. Rapid response preparedness for management of post-covid invasive rhino-orbito-cerebral fungal infections: experience from a tertiary care government institute in India. Indian J Otolaryngol Head Neck Surg. 2021. p. 1-3
9. Hosseini SM, Borghei P. Rhinocerebral mucormycosis: Pathways of spread. Eur Arch Otorhinolaryngol. 2005. 262: 932-8
10. John TM, Jacob CN, Kontoyiannis DP. When uncontrolled diabetes mellitus and severe COVID-19 converge: The perfect storm for mucormycosis. J Fungi (Basel). 2021. 7: 298
11. Mitra S, Janweja M, Sengupta A. Post-COVID-19 rhino-orbito-cerebral mucormycosis: A new addition to challenges in pandemic control. Eur Arch Otorhinolaryngol. 2022. 279: 2417-22
12. Orguc S, Yücetürk AV, Demir MA, Goktan C. Rhinocerebral mucormycosis: Perineural spread via the trigeminal nerve. J Clin Neurosci. 2005. 12: 484-6
13. Pal P, Singh B, Singla S, Kaur R. Mucormycosis in COVID-19 pandemic and its neurovascular spread. Eur Arch Otorhinolaryngol. 2022. 279: 2965-72
14. Pal R, Singh B, Bhadada SK, Banerjee M, Bhogal RS, Hage N. COVID-19-associated mucormycosis: An updated systematic review of literature. Mycoses. 2021. 64: 1452-9
15. Patil A, Mohanty HS, Kumar S, Nandikoor S, Meganathan P. Angioinvasive rhinocerebral mucormycosis with complete unilateral thrombosis of internal carotid artery-case report and review of literature. BJR Case Rep. 2016. 2: 20150448
16. Popli H, Gupta A, Singh V, Agarwal V, Akilan R, Kumar A. Are low serum Vitamin D levels a risk factor for advent of COVID-19 associated rhinocerebral mucormycosis: A preliminary case control study. Indian J Otolaryngol Head Neck Surg. 2022. p. 1-5
17. Rudrabhatla PK, Reghukumar A, Thomas SV. Mucormycosis in COVID-19 patients: Predisposing factors, prevention and management. Acta Neurol Belg. 2022. 122: 273-80
18. Sen M, Honavar SG, Bansal R, Sengupta S, Rao R, Kim U. Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India collaborative OPAI-IJO study on mucormycosis in COVID-19 (COSMIC), report 1. Indian J Ophthalmol. 2021. 69: 1670-92
19. Sharma A, Goel A. Mucormycosis: Risk factors, diagnosis, treatments, and challenges during COVID-19 pandemic. Folia Microbiol (Praha). 2022. 67: 363-87
20. Sharma K, Tiwari T, Goyal S, Sharma R. Rhino-orbito-cerebral mucormycosis causing cranial nerve abscess in post-COVID-19 status. BMJ Case Rep. 2021. 14: e245756
21. Singh V, Singh M, Joshi C, Sangwan J. Rhinocerebral mucormycosis in a patient with Type 1 diabetes presenting as toothache: A case report from Himalayan region of India. BMJ Case Rep. 2013. 2013: bcr2013200811
22. Soni K, Das A, Sharma V, Goyal A, Choudhury B, Chugh A. Surgical and medical management of ROCM (Rhino-orbito-cerebral mucormycosis) epidemic in COVID-19 era and its outcomes a tertiary care center experience. J Mycol Med. 2022. 32: 101238
23. Sundaram N, Bhende T, Yashwant R, Jadhav S, Jain A. Mucormycosis in COVID-19 patients. Indian J Ophthalmol. 2021. 69: 3728-33
24. Vaid N, Mishra P, Gokhale N, Vaid S, Vaze V, Kothadiya A. A proposed grading system and experience of COVID-19 associated rhino orbito cerebral mucormycosis from an Indian tertiary care center. Indian J Otolaryngol Head Neck Surg. 2021. p. 1-8
25. Verma V, Acharya S, Kumar S, Gaidhane SA, Thatere U. Rhinocerebral mucormycosis with brain abscess presenting as status epileptucus in a COVID-19-infected male: A calamitous complication. Cureus. 2022. 14: e21061
26. Vogt N, Heß K, Bialek R, Buerke B, Brüggemann M, Topp MS. Epileptic seizures and rhinocerebral mucormycosis during blinatumomab treatment in a patient with biphenotypic acute leukemia. Ann Hematol. 2017. 96: 151-3