- Department of Neurosurgery, Fayoum University Hospitals, Fayoum University, Fayoum, Egypt.
- Department of Neurosurgery, Cairo University, Cairo, Cairo, Egypt.
Correspondence Address:
Ahmed Hosameldin, Department of Neurosurgery, Fayoum University Hospitals, Fayoum University, Fayoum, Egypt.
DOI:10.25259/SNI_1236_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ahmed Hosameldin1, Mohammed Hussein1, Ehab Abdelhalim2, Mohammed Shehab2, Ashraf Osman1. Surgical management of spontaneous thoracic and lumbar spondylodiscitis by fixation and debridement. 11-Feb-2022;13:44
How to cite this URL: Ahmed Hosameldin1, Mohammed Hussein1, Ehab Abdelhalim2, Mohammed Shehab2, Ashraf Osman1. Surgical management of spontaneous thoracic and lumbar spondylodiscitis by fixation and debridement. 11-Feb-2022;13:44. Available from: https://surgicalneurologyint.com/surgicalint-articles/11386/
Abstract
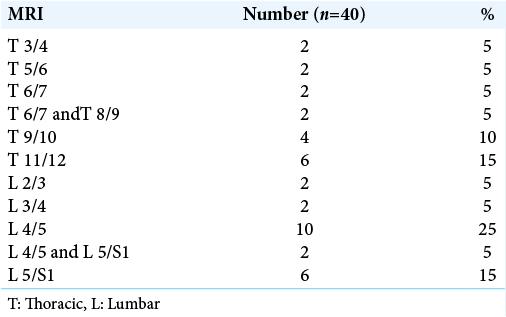
Background: Spondylodiscitis could be considered one of the most disturbing challenges that face neurosurgeons due to variety of management strategies. The lumbar region was highly affected then dorsal region with higher percentage for lesion in L4/5 (25%) followed by T11/12 and L5/S1 (15%). In our study, we discuss the efficacy of debridement and fixation in cases of spontaneous thoracic and lumbar spondylodiscitis.
Methods: This retrospective study included 40 patients with spontaneous thoracic or lumbar spondylodiscitis indicated for surgical intervention in the period from March 2019 to February 2021. All patients were subjected to thorough history taking, neurological examination, and investigations. The patients were operated on through posterior approach by debridement and posterior transpedicular screws fixation and fusion.
Results:
Conclusion:
Keywords: Debridement, Fixation, Lumbar, Spondylodiscitis, Spontaneous, Thoracic
INTRODUCTION
Spinal infections encompass several entities that have characteristic presentations and clinical courses. These are often pyogenic but can also be granulomatous or parasitic and include spondylodiscitis, septic discitis, vertebral osteomyelitis, and epidural abscess.[
Debridement without fusion is advocated by some surgeons, however, large multilevel laminectomies without instrumentation may result in severe instability and provoke neurological deficit.[
The aim of our study is to evaluate the impact of debridement and fixation on clinical and radiological outcomes in patients of spontaneous spondylodiscitis.
MATERIALS AND METHODS
This retrospective study included 40 patients with spontaneous thoracic or lumbar spondylodiscitis indicated for surgical intervention in the period from March 2019 to February 2021. Age ranged between 15 and 75 years old and all patients with spontaneous spondylodiscitis in thoracic or lumbar regions. All patients were subjected to thorough history taking, neurological examination, and investigations.
Investigations included laboratory in the form of full routine laboratories with special concern to erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). Furthermore, radiological (plain X-rays and magnetic resonance imaging [MRI]) of affected spine region was performed to assess the neural elements with good visualization of neural canal components, intervertebral discs, and computed tomography (CT) of pathological spine was done. It provides accurate details of bony anatomy, diameter of bony canal and allows measurement of the length of the screws. It is useful for the assessment of deformities and loss in vertebral height. The patients were operated on through posterior approach by debridement and posterior transpedicular screws fixation (long segment if affected level was at thoracolumbar junction while short segment if other single level lumbar or thoracic affection) and posterolateral fusion. Postoperative antibiotics for 28 days, analgesics, and neurotropic drugs were routinely used for all patients. The patients were followed up at inpatient and outpatient basis. Early follow-up (1st month postoperative) included postoperative back pain visual analog scale (VAS score), neurological status, radiological evaluation of placement of the fixation system, and alignment of the vertebral column.
Later follow-up (at 3 and 6 months) was divided into functional outcome which is improvement in back pain, laboratory and microbiological outcome with improving ESR&CRP laboratories, postoperative culture of the specimen, radiological outcome such as confirmation of incidence of fusion (after 6 months) and postoperative complications in the form of persistent infection, wound infection or implant failure, or pseudoarthrosis should be documented.
Statistical analysis
Data were collected, coded, and entered into Microsoft access. Data analysis was performed using Statistical Package for the Social Sciences-Version 20. The mean and standard deviation of assessed variable were presented. Categorical variables were expressed as numbers and percentages. Qualitative data were tested for normality using one-sample Kolmogorov–Smirnov test. Mann–Whitney U-test was used to compare two independent groups and Chi-square test was used to compare more than 2 groups. P ≤ 0.05 was considered the cutoff point for statistical significance.
RESULTS
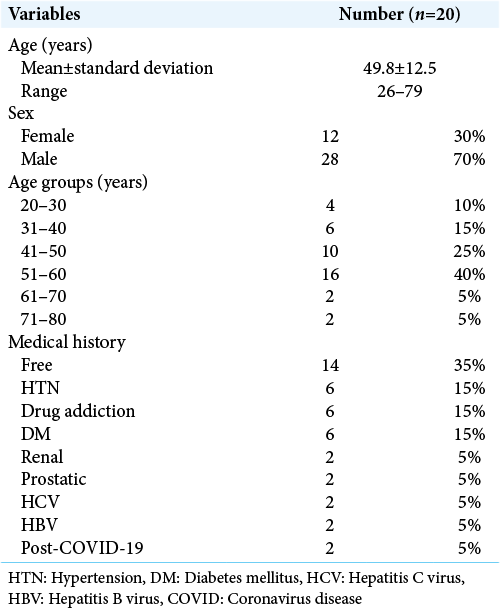
The mean age was 49.8 ± 12.5 years old ranged between 15 and 75 years old, as regard sex distribution, 70% were male, and 30% were female. As regard comorbidities, higher percentage was for diabetes mellitus (15%) and drug addiction (15%), other comorbidities included post-COVID, renal, hepatic, and prostatic cancer versus 35% were free [
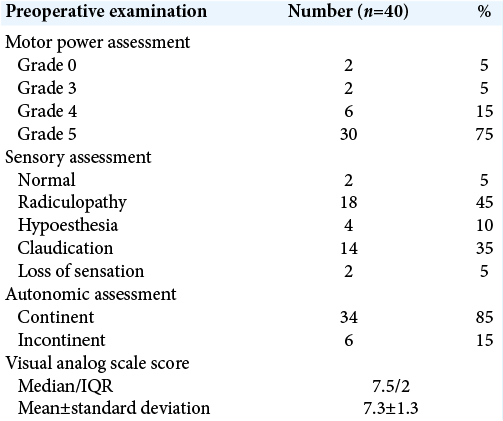
All cases had elevated ESR preoperative with 95% had positive CRP. Early postoperative 75% of cases were full motor power and 20% showed improvement in motor power, for sensory assessment, 85% showed improvement, with 85% were continent in autonomic (sphincteric) assessment and 10% showed improvement after operation.
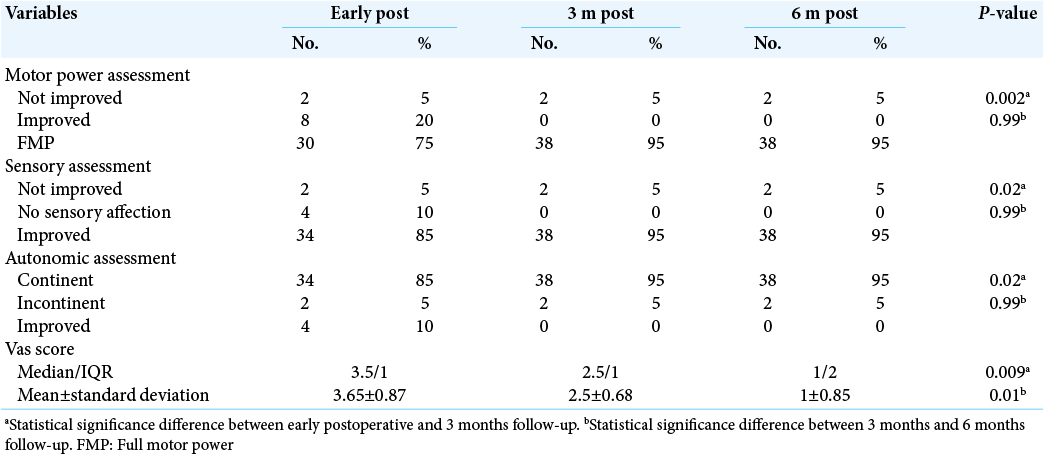
The mean VAS score was of 3.65 ± 0.87. After 3 months postoperative, the clinical assessment showed that 95% of cases were full motor power, for sensory assessment, 95% showed improvement, with 95% were continent in autonomic assessment. The mean VAS score was 2.5 ± 0.68. Culture results 65% of samples showed negative culture, 15% had methicillin-resistant Staphylococcus aureus (MRSA), and 10% had Escherichia coli results [
Six months postoperative, the clinical assessment showed that 95% of cases were full motor power, for sensory assessment, 95% showed improvement, with 95% were continent. The mean VAS score was 1 ± 0.85. After operation, 90% of cases showed improvement in ESR and 95% showed improvement in CRP. In our study, there was a statistical significance difference in motor power, sensory, and autonomic assessment in addition to VAS score between early postoperative and after 3 months follow-up with P < 0.05, which indicated proper improvement after 3 months. On the other hand, there was no statistical significance difference with P > 0.05 between clinical assessment after 3 and 6 months follow-up [
DISCUSSION
Spondylodiscitis is the most frequent among spinal infections. Titanium is the implant material of choice because it is very difficult for bacterial biofilm compared to other materials.[
Patients who underwent surgical treatment were significantly younger (mean age at presentation: 52.7 years) than patients who were medically treated (mean age at presentation: 64.8 years). The male: female ratio was 2.06:1.[
Mohamed et al. mentioned that low back pain was present in 22 cases (88%), 16 cases (64%) had lower limb pain (sciatic and claudication pain), and 12 cases (48%) had weakness.[
In our series, we performed surgical removal of the infected tissue and we did not use any surgical site local antibiotics. While Fleege et al. recommended surgical removal of the infection by extensive debridement with stabilization and filling the defect with a high local dose of antibiotic and a mixture of cancellous bone and antibiotic-loaded hydroxyapatite and calcium sulfate.[
Eight conservatively managed spondylodiscitis cases were associated with a history of proven pulmonary tuberculosis. Choi et al. and Mohamed et al. revealed that the most common accused bacteria were S. aureus, while Waheed et al., Mycobacterium tuberculosis was the most frequent organism isolated (15 patients), followed by pyogenic infection (six patients with brucellosis, three with S. aureus, and three with streptococcal infection). However, they failed to obtain microbiological confirmation in 17 cases (38.6%) despite performing multiple cultures. Postoperative 90% of our cases showed improvement in ESR levels and 95% showed improvement in CRP results. Furthermore, Noha et al., Kamal et al., Fleege et al., and Tsai et al. showed the same finding. Our study agreed with Ozlay et al., Blondel et al., and Zhang et al. who documented that bony union was obtained in all cases in a variable period ranged from 6 months to 2 years. Si et al. found good fusion in both study groups and radiological results showed no significant difference in fusion time between them.[
In our study, there were 65% of cases showed no complications but 20% had wound infection (eight cases) managed by repeated dressing with the targeted or empirical antibiotic as reported in the antibiogram and the eight cases showed improvement in the wound condition without need for another return to operative theater for redebridement or implants removal, 10% had delay in fusion with a single case of implant pull out before full fusion. Mohamed et al. recorded single case of fixation looseness and another one for early superficial wound infection.[
CONCLUSION
In our study, we managed patients of spontaneous thoracic and lumbar spondylodiscitis by surgical debridement with instrumentation through posterolateral open transpedicular fixation; it seems to be effective and safe. We found that the clinical condition of our patients showed significant improvement with this addressed approach and it also allows effective and rapid cure of inflammation, earlier ambulation, and significantly shorter duration of antibiotic usage.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bailey HL, Gabriel M, Hodgson AR, Shin JS. Tuberculosis of the spine in children. Operative findings and results in one hundred consecutive patients treated by removal of the lesion and anterior grafting. J Bone Joint Surg Am. 1972. 54: 1633-57
2. Blondelg B, Fuentes S, Pech-Gourg G, Metellus P, Dufour H. Minimally invasive osteosynthesis in septic conditions. Neurochirurgie. 2011. 57: 15-20
3. Butler JS, Shelly MJ, Timlin M, Powderly WG, O’Byrne JM. Nontuberculous pyogenic spinal infection in adults: A 12-year experience from a tertiary referral center. Spine (Phila Pa 1976). 2006. 31: 2695-700
4. Carragee EJ. Instrumentation of the infected and unstable spine: A review of 17 cases from the thoracic and lumbar spine with pyogenic infections. J Spinal Disord. 1997. 10: 317-24
5. Choi EJ, Kim SY, Kim HG, Kim TK, Kim KH, Shon HS. Percutaneous endoscopic debridement and drainage with four different approach methods for the treatment of spinal infection. Pain Physician. 2017. 20: E933-40
6. Dobran M, Lacoangeli M, Nasi D, Nocchi N, Colasant R, Vaira C. Posterior titanium screw fixation without debridement of infected tissue for the treatment of thoracolumbar spontaneous pyogenic spondylodiscitis. Asian Spine J. 2016. 10: 465
7. Duarte RM, Vaccaro AR. Spinal infection: State of the art and management algorithm. Eur Spine J. 2013. 22: 2787-99
8. Eismont FJ, Bohlman HH, Soni PL, Goldberg VM, Freehafer AA. Pyogenic and fungal vertebral osteomyelitis with paralysis. J Bone Joint Surg Am. 1983. 65: 19-29
9. Faraj AA, Webb JK. Spinal instrumentation for primary pyogenic infection report of 31 patients. Acta Orthop Belg. 2000. 66: 242-7
10. Fleege C, Wichelhaus TA, Rauschmann M. Systemic and local antibiotic therapy of conservative and operative treatment of spondylodiscitis. Orthopade. 2012. 41: 727-35
11. Gentile L, Benazzo F, de Rosa F, Boriani S, Dallagiacoma G, Franceschetti G. A systematic review: Characteristics, complications and treatment of spondylodiscitis. Eur Rev Med Pharmacol Sci. 2019. 23: 117-28
12. Giordan E, Marton E, Scotton G, Canova G. Outcomes and risk factors for spontaneous spondylodiscitis: Case series and meta-analysis of the literature. J Clin Neurosci. 2019. 68: 179-87
13. Gouliourisg T, Aliyu SH, Brown NM. Spondylodiscitis: Update on diagnosis and management. J Antimicrob Chemother. 2010. 65: iii11-24
14. Grados F, Lescure F, Senneville E, Flipo R, Schmit JL, Fardellone P. Suggestions for managing pyogenic (nontuberculous) discitis in adults. Joint Bone Spine. 2007. 74: 133-9
15. Griffith-Jones W, Nasto LA, Pola E, Stokes OM, Mehdian H. Percutaneous suction and irrigation for the treatment of recalcitrant pyogenic spondylodiscitis. J Orthop Traumatol. 2018. 19: 10
16. Guerado E, Cerván AM. Surgical treatment of spondylodiscitis. An update. Int Orthop. 2012. 36: 413-20
17. Hadjipavlou AG, Mader JT, Necessary JT, Muffoletto AJ. Hematogenous pyogenic spinal infections and their surgical management. Spine (Phila Pa 1976). 2000. 25: 1668-79
18. Hee HT, Majd ME, Holt RT, Pienkowski D. Better treatment of vertebral osteomyelitis using posterior stabilization and titanium mesh cages. J Spinal Disord Tech. 2002. 15: 149-56
19. Kamal AM, El-Sharkawi MM, El-Sabrout M, Hassan MG. Spondylodiscitis: Experience of surgical management of complicated cases after failed antibiotic treatment. SICOT J. 2020. 6: 5
20. Kuklo TR, Potter BK, Bell RS, Moquin RR, Rosner MK. Single-stage treatment of pyogenic spinal infection with titanium mesh cages. J Spinal Disord Tech. 2006. 19: 376-82
21. Landi A, Grasso G, Iaiani G, Gregori F, Mancarella C, di Bartolomeo A. Spontaneous spinal discitis and spondylodiscitis: Clinicotherapeutic remarks. J Neurosci Rural Pract. 2017. 8: 642
22. Lee JS, Suh KT. Posterior lumbar interbody fusion with an autogenous iliac crest bone graft in the treatment of pyogenic spondylodiscitis. J Bone Joint Surg Br. 2006. 88: 765-70
23. Lee MC, Wang MY, Fessler RG, Liauw J, Kim DH. Instrumentation in patients with spinal infection. Neurosurg Focus. 2004. 17: E7
24. Lu DS, Wang V, Chou D. The use of allograft or autograft and expandable titanium cages for the treatment of vertebral osteomyelitis. Neurosurgery. 2009. 64: 122-9
25. Mohamed AA, Soffar HM, El Zayat HH, Aboul-Ela HM. Prognosis of spinal infections managed by minimal debridement: A case series in two tertiary centers. Surg Neurol Int. 2021. 12: 83
26. Noh SH, Zhang HY, Lim HS, Song HJ, Yang KH. Decompression alone versus fusion for pyogenic spondylodiscitis. Spine J. 2017. 17: 1120-6
27. Ozalay M, Sahin O, Derincek A, Onay U, Turunc T, Uysal M. Non-tuberculous thoracic and lumbar spondylodiscitis: Single-stage anterior debridement and reconstruction, combined with posterior instrumentation and grafting. Acta Orthop Belg. 2010. 76: 100-6
28. Patel SS, Wilson A, Serkan I, Akpolat YT, Botimer GD, Cheng WK. A comparison of Staphylococcus aureus biofilm formation on cobalt-chrome and titanium-alloy spinal implants. J Clin Neurosci. 2016. 31: 219-23
29. Pourtaheri S, Kimona I, Tyler S, Eiman S, Remi A, Buerba RA. Comparison of instrumented and noninstrumented surgical treatment of severe vertebral osteomyelitis. Orthopedics. 2016. 39: e504-8
30. Ramey WL, von Glinski A, Jack A, Blecher R, Oskouian RJ, Chapman JR. Antibiotic-impregnated polymethylmethacrylate strut graft as a treatment of spinal osteomyelitis: Case series and description of novel technique. J Neurosurg Spine. 2020. 33: 415-20
31. Rand C, Smith MA. Anterior spinal tuberculosis: Paraplegia following laminectomy. Ann R Coll Surg Engl. 1989. 71: 105-9
32. Si M, Yang ZP, Li ZF, Yang Q, Li JM. Anterior versus posterior fixation for the treatment of lumbar pyogenic vertebral osteomyelitis. Orthopedics. 2013. 36: 831-6
33. Sudprasert W, Piyapromdee U, Lewsirirat S. Neurological recovery determined by C-reactive protein, erythrocyte sedimentation rate and two different posterior decompressive surgical procedures: A retrospective clinical study of patients with spinal tuberculosis. J Med Assoc Thai. 2015. 98: 993-1000
34. Sundararaj G, Amritanand R, Venkatesh K, Arockiaraj J. The use of titanium mesh cages in the reconstruction of anterior column defects in active spinal infections: Can we rest the crest?. Asian Spine J. 2011. 5: 155-61
35. Tsai TT, Yang SC, Niu CC, Lai PL, Lee ML, Chen LH. Early surgery with antibiotics treatment had better clinical outcomes than antibiotics treatment alone in patients with pyogenic spondylodiscitis: A retrospective cohort study. BMC Musculoskelet Disord. 2017. 18: 175
36. Turel MK, Kerolus M, Deutsch H. The role of minimally invasive spine surgery in the management of pyogenic spinal discitis. J Craniovertebr Junction Spine. 2017. 8: 39-43
37. Vergne P, Trèves R. Infectious spondylodiscitis. Etiology diagnosis, progression and treatment. Rev Prat. 1998. 48: 2065-71
38. Waheed G, Soliman MA, Ali AM, Aly MH. Spontaneous spondylodiscitis: Review, incidence, management, and clinical outcome in 44 patients. Neurosurg Focus. 2019. 46: E10
39. Yong HP, Jong DP, Choi YG, Lee SH. Anterior debridement and fusion followed by posterior pedicle screw fixation in pyogenic spondylodiscitis: Autologous iliac bone strut versus cage. J Neurosurg Spine. 2008. 8: 405-12
40. Zaveri CR, Mehta SS. Surgical treatment of lumbar tuberculous spondylodiscitis by transforaminal lumbar interbody fusion (TLIF) and posterior instrumentation. J Spinal Disord Tech. 2009. 22: 257-62
41. Zhang HQ, Guo CF, Xiao XG, Long WR, Deng ZS, Chen J. One-stage surgical management for multilevel tuberculous spondylitis of the upper thoracic region by anterior decompression, strut autografting, posterior instrumentation, and fusion. J Spinal Disord Tech. 2007. 20: 263-7