- Department of Neurosurgery, SUNY Upstate, Syracuse, New York, United States.
- Department of Pathology, SUNY Upstate, Syracuse, New York, United States.
Correspondence Address:
Haydn Hoffman
Department of Neurosurgery, SUNY Upstate, Syracuse, New York, United States.
DOI:10.25259/SNI_752_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Harman Chopra1, Haydn Hoffman1, Timothy E. Richardson2, Michael A. Galgano1. Surgical management of symptomatic vertebral hemangiomas: A case report and literature review. 17-Feb-2021;12:56
How to cite this URL: Harman Chopra1, Haydn Hoffman1, Timothy E. Richardson2, Michael A. Galgano1. Surgical management of symptomatic vertebral hemangiomas: A case report and literature review. 17-Feb-2021;12:56. Available from: https://surgicalneurologyint.com/surgicalint-articles/10597/
Abstract
Background: Vertebral hemangiomas (VHs) are common benign tumors that only rarely become symptomatic. There is a paucity of data regarding their surgical management and outcomes. Here, we reported a case involving an aggressive cervical VH, discussed its surgical management and outcomes, and reviewed the literature.
Methods: We assessed the clinical, radiological, and surgical outcomes for a patient with an aggressive cervical VH. We also performed a systematic review of the literature according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines to describe surgical outcomes for symptomatic VH.
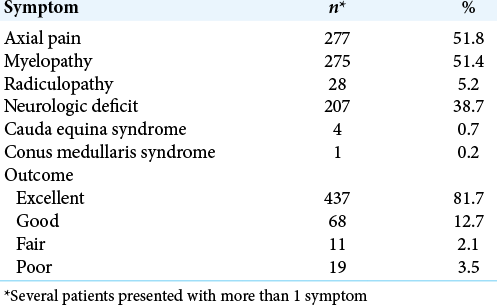
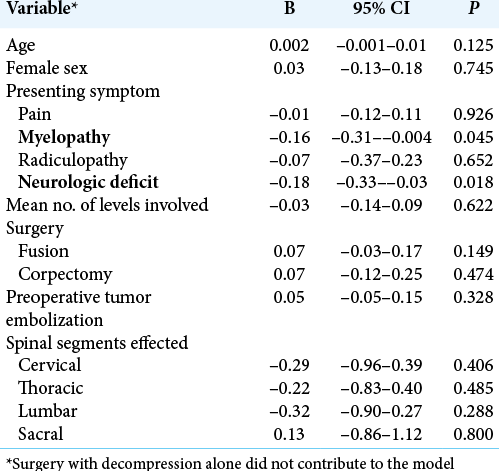
Results: A total of 154 studies including 535 patients with VH were included in the study. The majority of patients were female (62.8%), the average age was 43 years, and the thoracic spine was most commonly involved (80.6%). Utilizing Odom’s criteria, outcomes were excellent in 81.7% (95% CI 73.2–90.2) of cases. For those presenting with myelopathy (P = 0.045) or focal neurological deficits (P = 0.018), outcomes were less likely to be excellent. Preoperative embolization was not associated with excellent outcome (P = 0.328).
Conclusion: Surgical outcomes for VH are predominantly favorable, but aggressive VHs have the potential to cause significant residual postoperative neurological morbidity.
Keywords: Preoperative embolization, Spine tumor, Surgery, Vascular tumor, Vertebral hemangioma
INTRODUCTION
Vertebral hemangiomas (VHs) are benign vascular tumors comprised capillaries and venous structures. They have a prevalence of 10–12%, are usually asymptomatic, and rarely require surgery.[
Illustrative case
Presentation
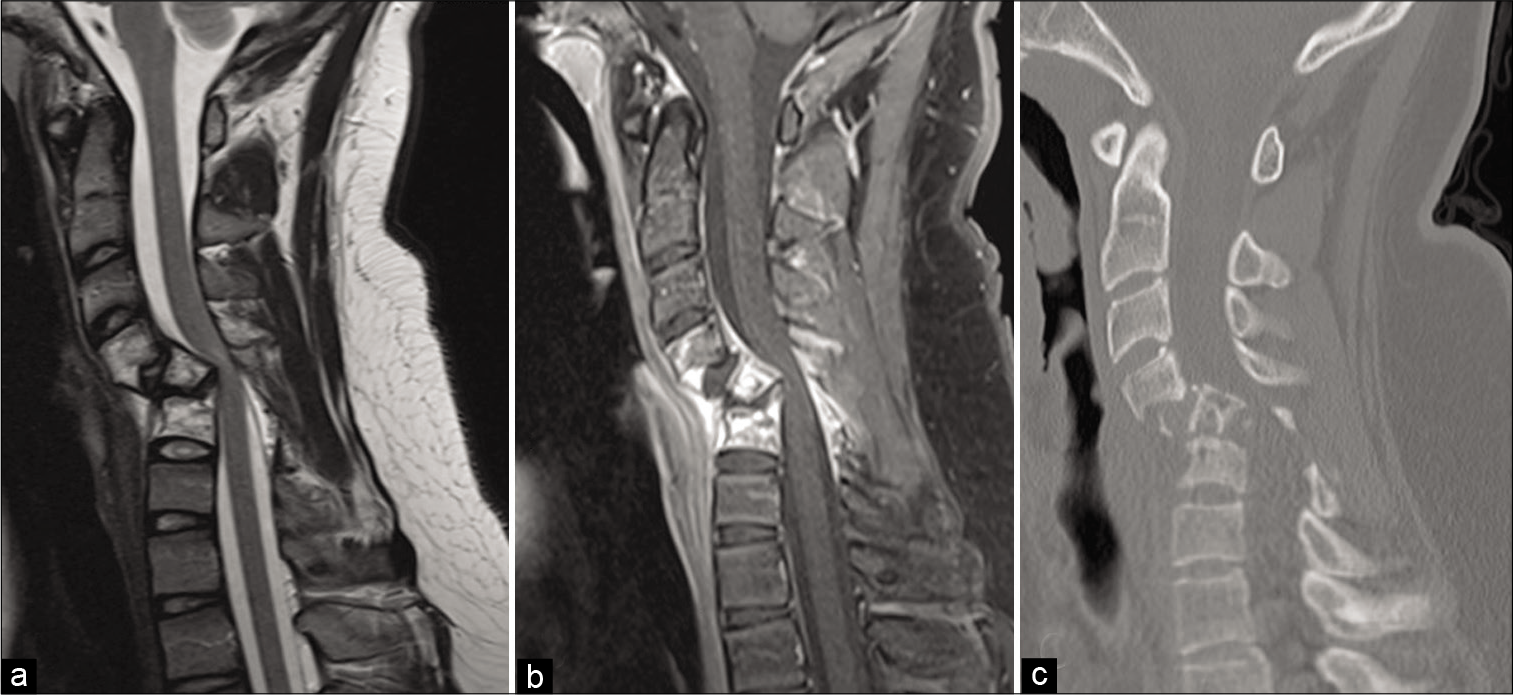
A 14-year-old male presented with 12 weeks of mechanical neck pain, hand weakness, and distal upper extremity paresthesias. MRI revealed abnormal anterior/posterior bony element enhancement from C4–C6 with subtle epidural enhancement [
Figure 1:
Preoperative sagittal T2-weighted (a) MRI demonstrates severe angulation and retropulsion of the C5 vertebral body causing marked spinal stenosis with hyperintensity within the spinal cord. Contrast-enhanced T1-weighted image (b) shows abnormal enhancement within the C4–C6 vertebral bodies, lamina, and spinous processes, as well as enhancement in the posterior epidural space with prominent flow voids. Plain sagittal CT obtained on presentation to the emergency department (c) demonstrates severe osteolysis at the levels of C4–C6 and increased retrolisthesis at C5.
Operation
The patient underwent an emergent C3–C7 anterior corpectomy with fusion. The C4, C5, and C6 vertebral bodies were removed en bloc and an expandable cage was inserted into the corpectomy defect followed by a plate spanning C3–C7. The posterior longitudinal ligament was abnormally vascular. An additional C4–C6 laminectomy with posterior C2–T3 fusion was performed in the same setting.
Pathological findings
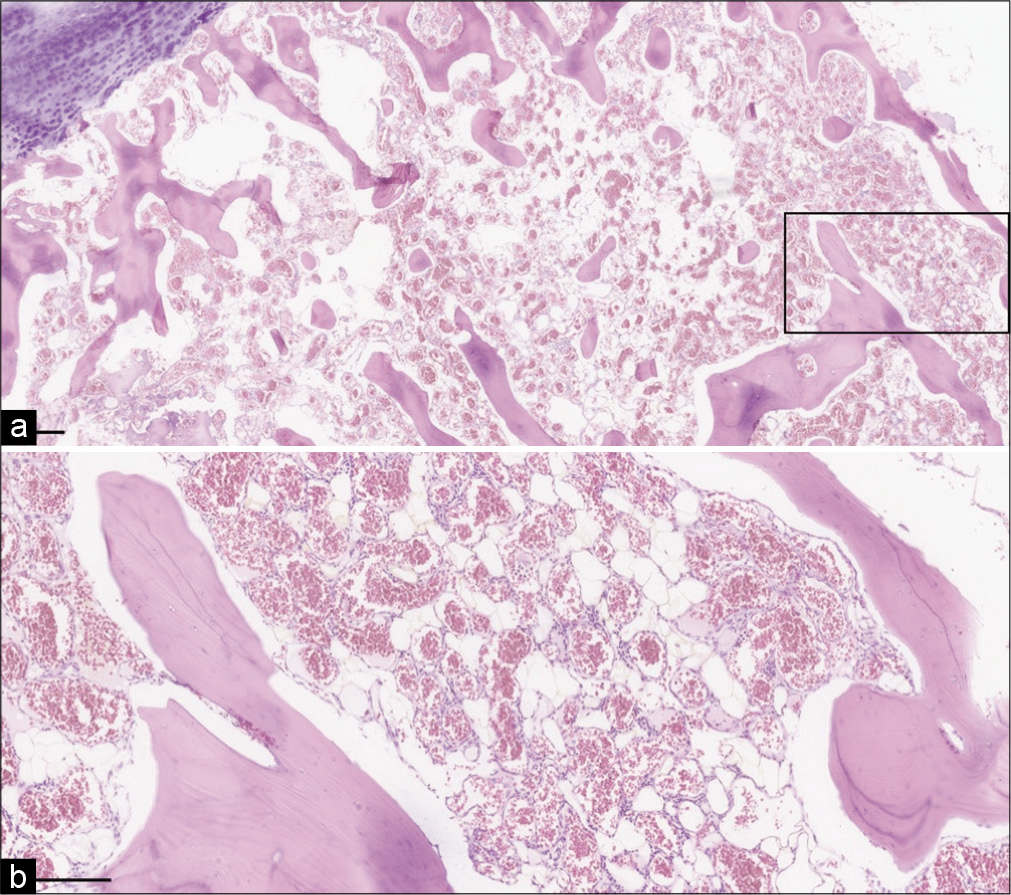
The histologic evaluation of the lesion [
Figure 2:
Microscopic sections demonstrate (a) mature cartilage and cortical bone with diffuse hemangioma replacement of bone marrow (total magnification = 20×, scale bar = 500 mm) and (b) higher power image demonstrates thin-walled vessels, focal adipose tissue, and minimal intervening stroma (total magnification = 100×, scale bar = 200 µm).
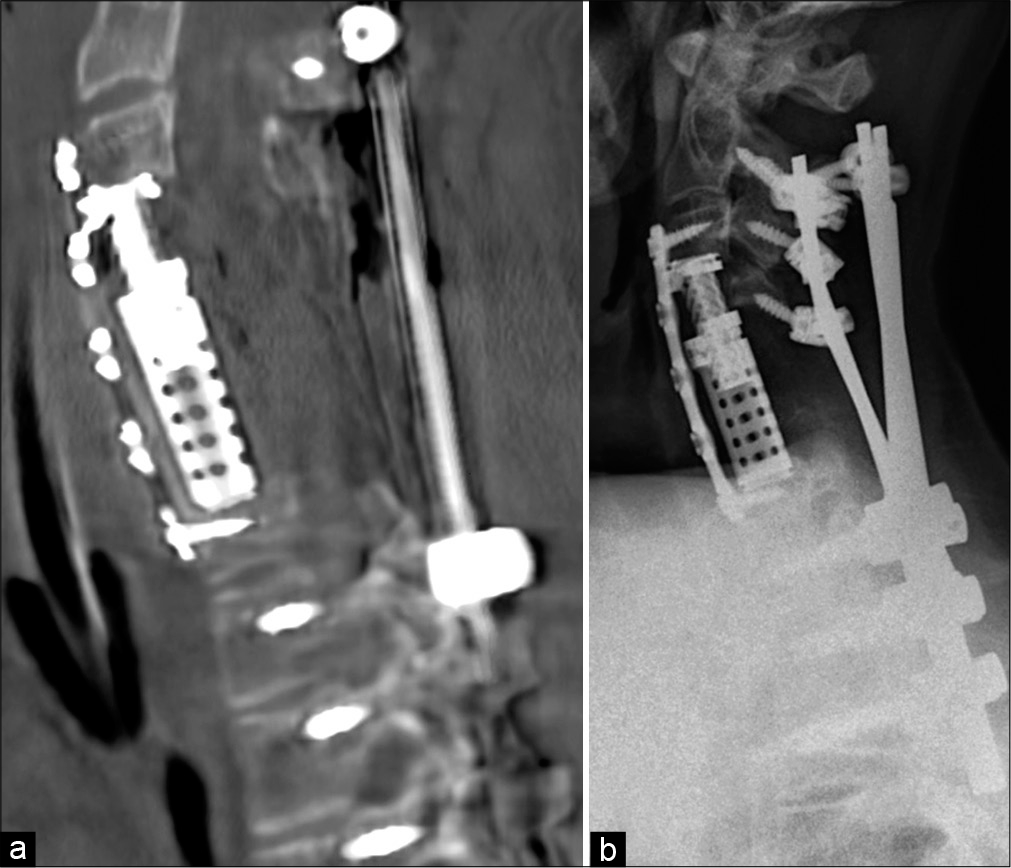
Postoperative course
The patient did not neurologically improve and was discharged to an inpatient rehabilitation center. Postoperative CT showed adequate decompression of the spinal canal, reduction of the C5 angulation, and correction of the kyphosis [
METHODS
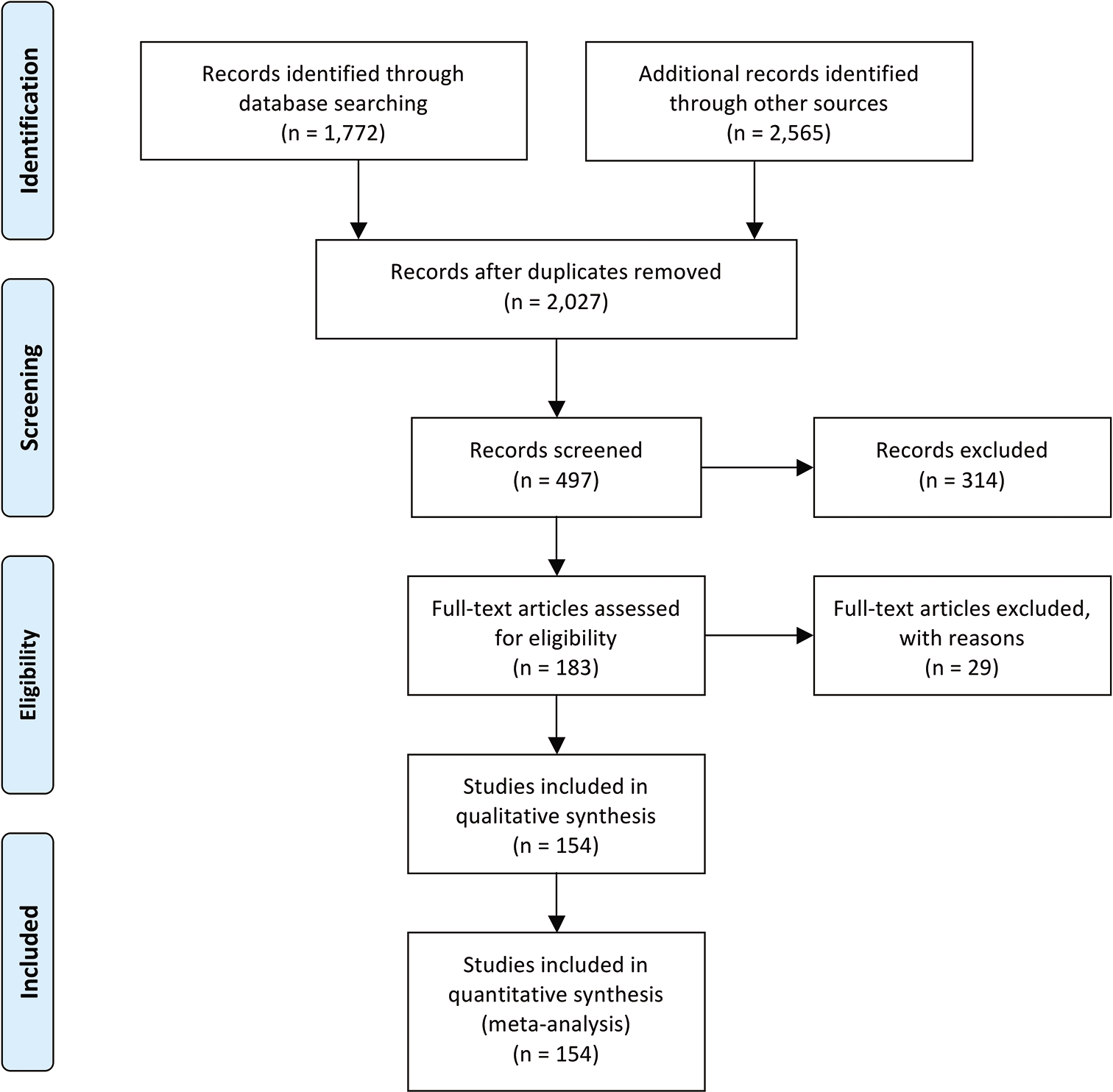
A systematic review was performed utilizing the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. MEDLINE, EMBASE, and Scopus were searched using the criteria “vertebral hemangioma” OR “spine hemangioma.” All case reports and series reporting outcomes after open surgery with or without instrumentation for symptomatic VH were included. Outcomes were categorized as excellent, good, fair, or poor according to Odom’s criteria. Random effects model was used to calculate the pooled rate of an excellent outcome. Weighted least squares regression was used to identify factors associated with an excellent outcome.
RESULTS
A total of 154 studies were included (1950–2020) comprising 535 patients [
DISCUSSION
Our case is unique for several reasons. Less than 6% of the cases in our review involved three or more levels as ours did. Only 3% involved the cervical spine. Presentation in a pediatric patient is also rare, with <15 cases reported worldwide. To the best of our knowledge, the only other report of a pediatric patient with an aggressive, symptomatic, multilevel VH involved the thoracic spine.[
The 535 patients undergoing surgery in this review displayed similar characteristics as seen in other VH series. We identified a female predominance, which may be due to the growth-stimulating effect of progesterone on the hemangioma. The median age in our analysis was 43 years, which is similar to other cohorts. The most common surgical technique was decompression without fusion. This was likely due to VHs predilection for the thoracic spine, which permits more aggressive decompression without instrumentation as the ribs provide stability. Although not included in our review, percutaneous vertebroplasty or radiotherapy is reasonable treatment options for patients presenting with only axial pain.[
Both myelopathy and neurologic deficit at presentation were negatively associated with excellent outcomes, suggesting that injury to neural elements is less likely to be reversed from surgery. Thus, early surgery before the development of neurologic compromise may be indicated for symptomatic VHs, especially if they display an epidural component. Further, there are multiple reports describing rapid progression of neurologic deficits.[
CONCLUSION
VHs can cause significant neurologic morbidity and outcomes after surgical intervention are predominantly favorable. The presence of preoperative myelopathy and neurologic deficit was negatively associated with an excellent outcome, suggesting that a prophylactic approach to symptomatic VHs is warranted.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abi-Fadel W, Afif N, Farah S, Haddad A, Rizk K, Raad J. Vertebral hemangioma symptomatic during pregnancy. A case report and review of the literature. J Gynecol Obstet Biol Reprod (Paris). 1997. 26: 90-4
2. Boschi V, Pogorelić Z, Gulan G, Perko Z, Grandić L, Radonić V. Management of cement vertebroplasty in the treatment of vertebral hemangioma. Scand J Surg. 2011. 100: 120-4
3. Cherian J, Sayama CM, Adesina AM, Lam SK, Luerssen TG, Jea A. Multilevel thoracic hemangioma with spinal cord compression in a pediatric patient: Case report and review of the literature. Childs Nerv Syst. 2014. 30: 1571-6
4. Cheung NK, Doorenbosch X, Christie JG. Rapid onset aggressive vertebral haemangioma. Childs Nerv Syst. 2011. 27: 469-72
5. Corniola MV, Schonauer C, Bernava G, Machi P, Yilmaz H, Lemée JM. Thoracic aggressive vertebral hemangiomas: Multidisciplinary management in a hybrid room. Eur Spine J. 2020. 29: 3179-86
6. Fox MW, Onofrio BM. The natural history and management of symptomatic and asymptomatic vertebral hemangiomas. J Neurosurg. 1993. 78: 36-45
7. Lang EF, Peserico L. Neurologic and surgical aspects of vertebral hemangiomas. Surg Clin North Am. 1960. 40: 817-23
8. Macki M, Bydon M, Kaloostian P, Bydon A. Acute compressive myelopathy due to vertebral haemangioma. BMJ Case Rep. 2014. 2014: bcr2013200408
9. Newmark J, Jones HR, Thomas CB, Aretz HT, Freiberg SR, Baker RA. Vertebral haemangioma causing acute recurrent spinal cord compression. J Neurol Neurosurg Psychiatry. 1991. 54: 471
10. Tarantino R, Donnarumma P, Nigro L, Delfini R. Surgery in extensive vertebral hemangioma: Case report, literature review and a new algorithm proposal. Neurosurg Rev. 2015. 38: 585-92
11. Templin CR, Stambough JB, Stambough JL. Acute spinal cord compression caused by vertebral hemangioma. Spine J. 2004. 4: 595-600
12. Trungu S, Forcato S, Scollato A, Miscusi M, Raco A. Aggressive vertebral hemangioma causing acute spinal cord compression. J Neurosci Rural Pract. 2019. 10: 672-4
13. Vinay S, Khan SK, Braybrooke JR. Lumbar vertebral haemangioma causing pathological fracture, epidural haemorrhage, and cord compression: A case report and review of literature. J Spinal Cord Med. 2011. 34: 335-9