- Department of Neurosurgery, Dr. Alejandro Cabral Hospital, San Juan de la Maguana, Dominican Republic,
- Department of Neurosurgery, Peoples’ Friendship University of Russia, Moscow, Russia,
- Laboratory of Microsurgical Neuroanatomy, Second Chair of Gross Anatomy, School of Medicine, University of Buenos Aires, Argentina,
- Department of Anesthesiology, Dr. Alejandro Cabral Hospital, Diego De Velasquez, San Juan de la Maguana, Dominican Republic,
- Department of Veterinary Medicine, College of Agriculture and Veterinary Medicine, United Arab Emirates University, Al Ain, United Arab Emirates,
- Department of Spinal Surgery, Central Clinical Hospital of the Russian Academy of Sciences, Moscow, Russian Federation,
- One Health Center for Zoonoses and Tropical Veterinary Medicine, Ross University School of Veterinary Medicine, Basseterre, Saint Kitts and Nevis,
- Medical Student, Autonomous University of Santo Domingo, Santo Domingo, Dominican Republic.
Correspondence Address:
Andreina Rosario Rosario, Medical Student, Autonomous University of Santo Domingo, Santo Domingo, Dominican Republic.
DOI:10.25259/SNI_385_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ismael Peralta1, Manuel De Jesus Encarnación Ramírez2, Matias Baldoncini3, Dauly Vicente4, Arve Lee Willingham5, Renat Nurmukhametov6, Sandy Valdez1, Yussaira Castillo7, Daniel Antonio Encarnación2, Idelis Josefina Ramírez Soler1, Andreina Rosario Rosario8. Surgical nuances of giant neurocysticercosis according to intracranial location in the Southwest region of the Dominican Republic, presentation of cases, and literature review. 14-Jul-2023;14:242
How to cite this URL: Ismael Peralta1, Manuel De Jesus Encarnación Ramírez2, Matias Baldoncini3, Dauly Vicente4, Arve Lee Willingham5, Renat Nurmukhametov6, Sandy Valdez1, Yussaira Castillo7, Daniel Antonio Encarnación2, Idelis Josefina Ramírez Soler1, Andreina Rosario Rosario8. Surgical nuances of giant neurocysticercosis according to intracranial location in the Southwest region of the Dominican Republic, presentation of cases, and literature review. 14-Jul-2023;14:242. Available from: https://surgicalneurologyint.com/surgicalint-articles/12431/
Abstract
Background: Neurocysticercosis (NCC) is the most common infestation of the central nervous system, caused by the larval stage of the pig tapeworm Taenia solium. It is prevalent in regions with poor sanitation and underdevelopment, such as Latin America.
Case Description: We present four cases in which they harbored an intraventricular/intraparenchymal, frontal convexity, cerebellomedullary, and intraparenchymal NCC cyst of medium size, respectively. Three of them underwent complete removal of the cyst by craniotomy; the fourth had a shunt for obstructive hydrocephalus first, followed by excision of a suboccipital cyst 8 months later.
Conclusion: The intraventricular/intraparenchymal lesion was more complex to treat than its subarachnoid counterparts because the average brain should be transected and dissected away to achieve total removal. Waterjet dissection, arachnoid microdissection, and cyst drainage allowed minor brain damage than capsule coagulation and traction. Populated prospective studies are needed better to understand the surgical nuances of these rare entities.
Keywords: Cyst, Neurocysticercosis, Surgical technique, Taenia solium, Treatment
INTRODUCTION
Cysticercosis is a multi-system disease resulting from the seeding of the larval form of the pork tapeworm, Taenia solium, to various organs of the body. It is contracted through the fecal-oral route after ingesting viable eggs in foods contaminated with human feces.
T. solium is one of the seven neglected endemic zoonoses targeted by the WHO, and it is known to be endemic in broad areas of the world, including Latin America, Eastern Europe, sub-Saharan Africa, and parts of Asia, including the Indian subcontinent, Southeast Asia, and large regions of China.[
Some extensive studies have shown increasing incidence in developed countries such as the United States, and it is very variable around the world. In Latin America, the incidence varies depending on the urban or rural region, from 121.7 to 138.4 cases/100,000 individuals per year. Sanitary conditions have a close relationship with NCC, and combating this disease is a priority for the World Health Organization (WHO).[
NCC may cause focal epilepsy from its lesions or lead to distant epileptogenesis through the development of hippocampal sclerosis and subsequent mesial temporal lobe epilepsy. Across all causes of acquired epilepsy (epilepsy not associated with genetic causative mechanisms or delivery), NCC is likely the most frequent causative mechanism in low-income countries.[
NCC has been reported as the most frequent helminthic infection of the central nervous system (CNS). Yet, there have been very few studies conducted in the Caribbean region to estimate the prevalence of NCC. This is primarily because NCC can only be diagnosed with certainty through neuroimaging or autopsy. Hence, the frequency of sequelae following infection with the larval stages of T. solium remains largely unknown.[
METHODS
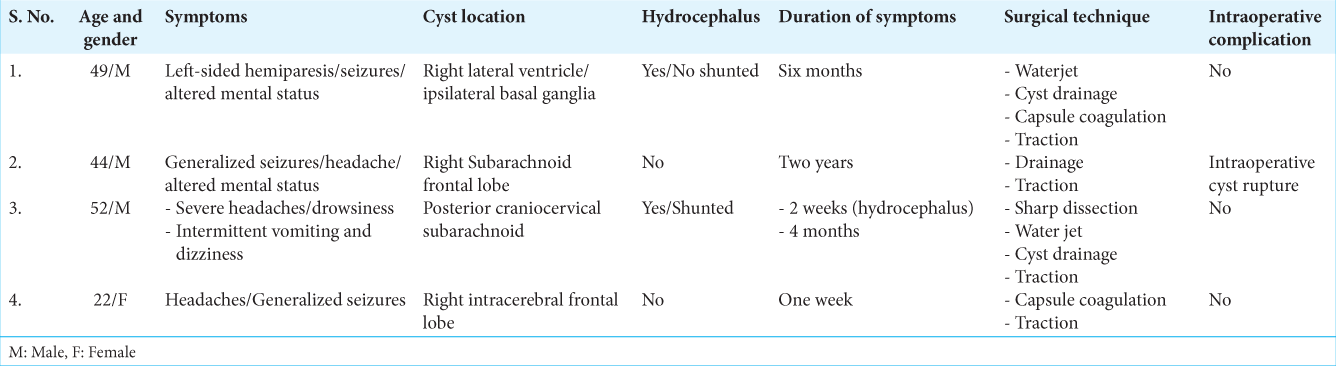
The present article is a retrospective case series and literature review. We identified four cases of patients with giant neurocysticerci who underwent surgical treatment at our institution between 2015 and 2020 in the south region of the Dominican Republic [
The results of the literature review were summarized narratively, with a focus on the surgical nuances of giant neurocysticerci according to intracranial location. This study was approved by the Institutional Review Board, and informed consent was obtained from all patients included in the case series.
CASE NO. 1
Clinical presentation and imaging
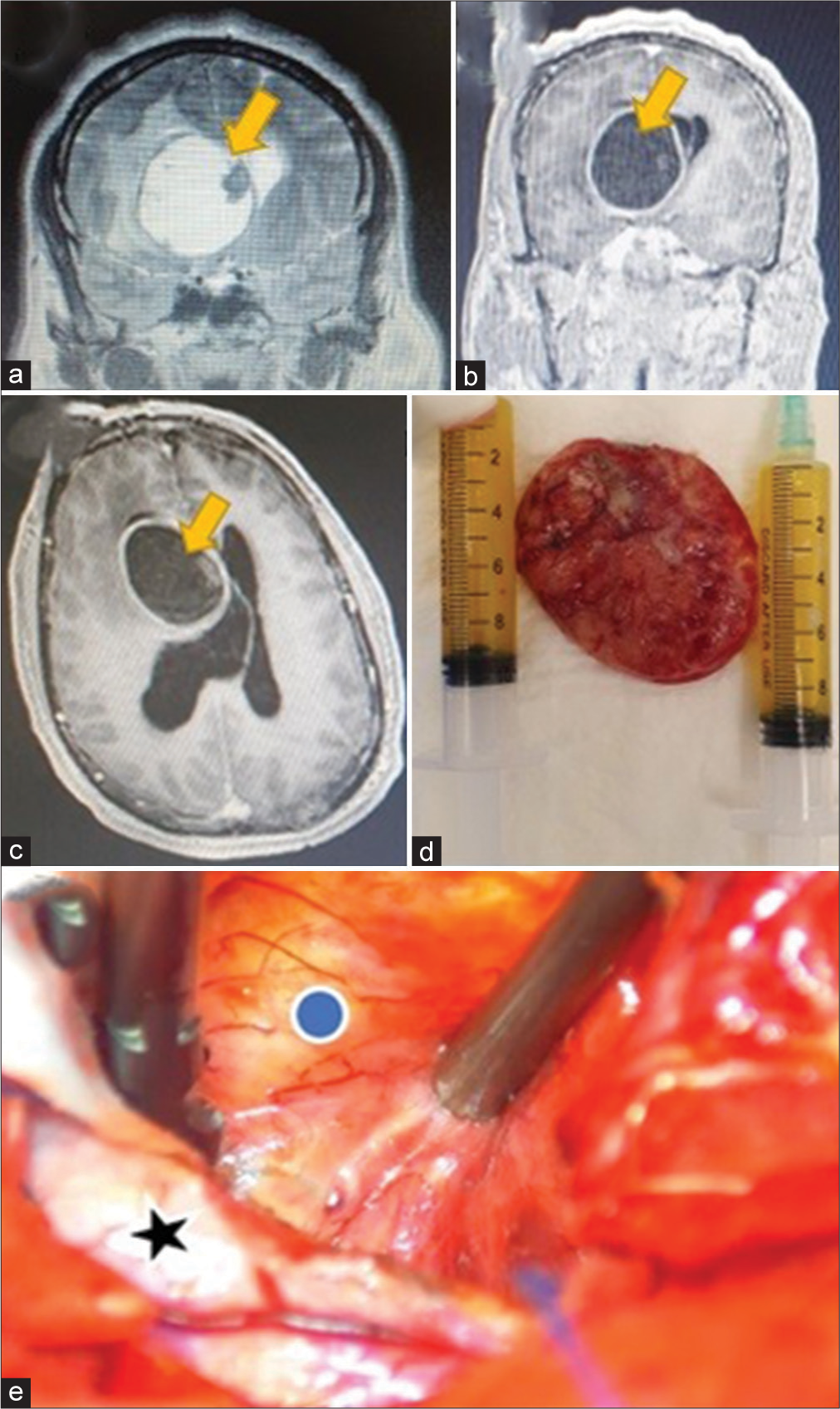
A 49-year-old male patient, obese, human immunodeficiency virus-positive (irregularly treated), presented to the emergency room (ER) with a 6-month history of altered mental status, left-sided hemiparesis (motor power grade 3/5), and left partial seizures. A computed tomography scan showed three cystic lesions, the biggest causing mass effect, in the right lateral ventricle extending to the ipsilateral basal ganglia. Contrast magnetic resonance imaging (MRI) showed a large (9.5 × 5.2 × 5.6 cm) enhancing cystic lesion with an enhancing nodule with substantial perilesional edema, compressing the ipsilateral ventricle and brain. The cyst was hypointense on the T1-weighted image and hyperintense on T2 and fluid-attenuated inversion recovery weighted image [
Figure 1:
(a and b) Coronal view, T2- and T1-contrasted sequence magnetic resonance imaging (MRI) showing the intraventricular cyst, with scolex (yellow arrow). (c) Axial T1-contrasted MRI, the yellow arrow shows the giant cyst. (d) Excised cyst with intracapsular fluid drained (syringes). (e) Intraoperative exoscopic view of the cyst (blue dot) and the adjacent brain parenchyma (black star).
Techniques
A right 6 × 7 cm frontal craniotomy was performed, and a superior frontal gyrus (F1) approach was used, transecting its posterior portion. The first step was waterjet dissection between the cyst and the surrounding brain and ventricle, allowing adhesions separation. Then, we drained 20 mL of a yellowish serous fluid, reducing the cyst volume. Bipolar coagulation shrank the capsule, and gentle traction of the lesion, protecting the edematous brain with patties, allowed the complete cyst excision. Histopathology confirmed the diagnosis of cysticercus cyst.
CASE NO. 2
Clinical presentation and imaging
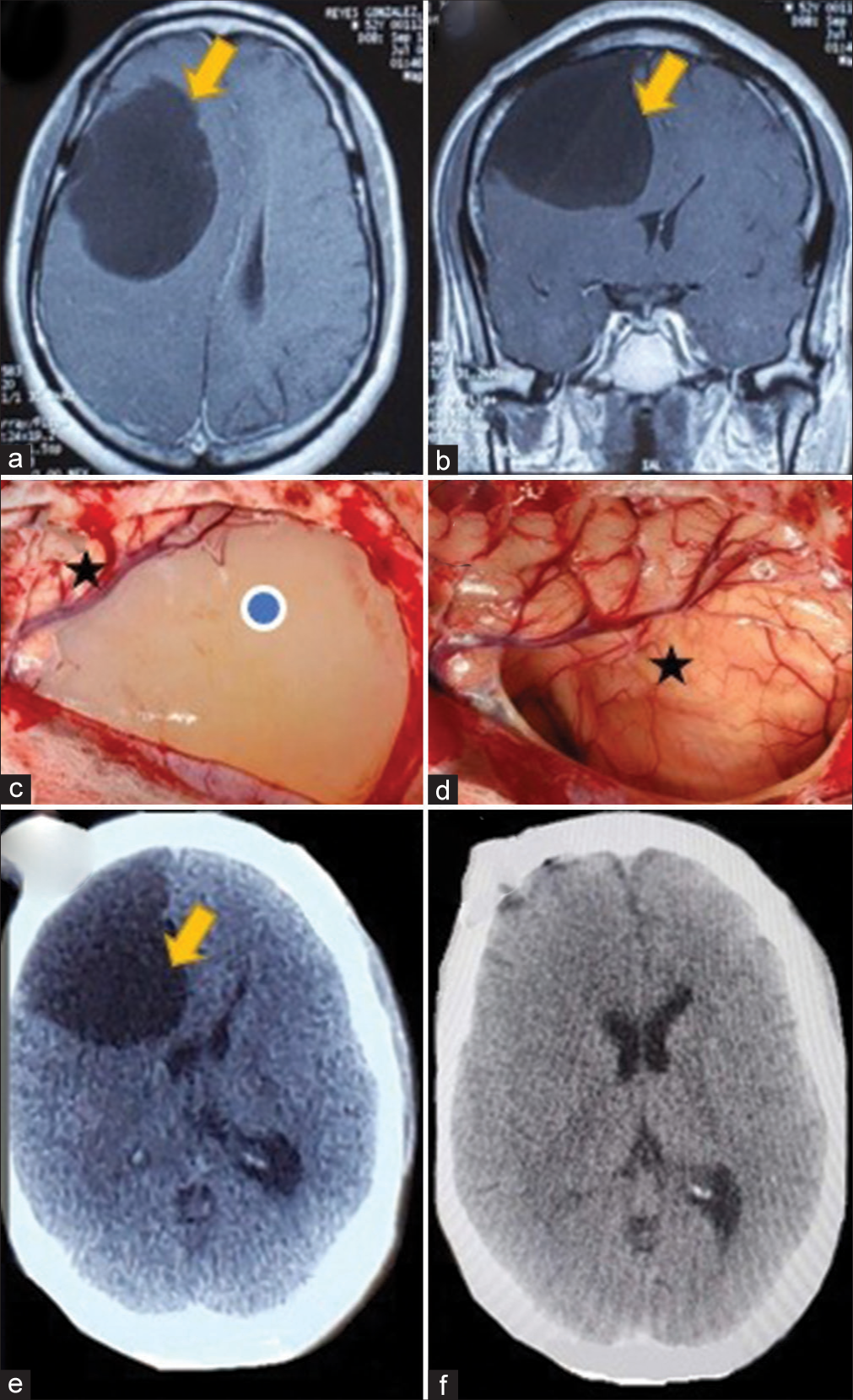
A 44-year-old male patient, treated for 2 years by a psychiatrist for a supposed bipolarity, was seen in the outpatient clinic presenting personality changes, headaches, and generalized seizures, the latter 2 months before presentation. Computed tomography (CT) scan showed a prominent (12 × 8 × 8 cm) irregular subarachnoid hypodense cystic lesion above the right frontal lobe with a critical mass effect. Contrast MRI demonstrated a nonenhancing capsule and nodule without perilesional edema [
Figure 2:
(a) Axial T1 contrast-weighted magnetic resonance imaging (MRI) with mass effect cyst (yellow arrow) and (b) coronal view, T1-contrasted sequence MRI showing the giant cyst (yellow arrow). (c) Intraoperative view of the cyst (blue dot) and adjacent brain (black star). (d) Intraoperative view of compressed brain after complete cyst removal (black star). (e) Preoperative axial view of a head computed tomography (CT) scan showing the compressing cyst (yellow arrow). (f) Postoperative head CT scan demonstrating the complete cyst removal.
Techniques
A right 10 × 7 cm frontal craniotomy was done. The cyst was immediately beneath the dura, and it had a thin capsule filled with white fluid. We tried sharp dissection first, between the lesion and brain, to develop a cleavage plane, but a spontaneous rupture occurred. Hence, we performed complete drainage followed by traction of the thin capsule.
CASE NO. 3
Clinical presentation and imaging
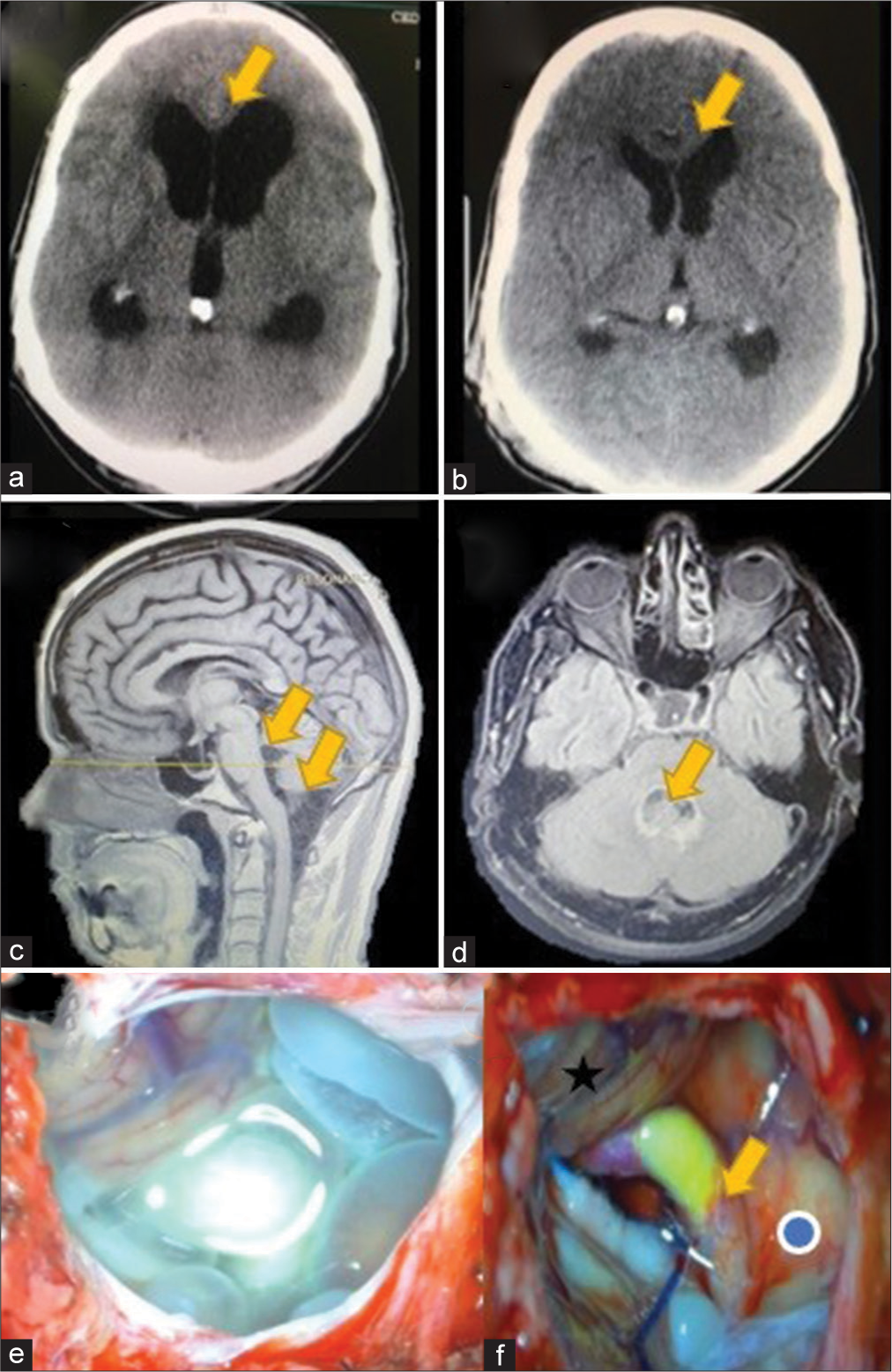
A 52-year-old male patient presented to the ER with a 2-week history of severe headaches. On examination, he was drowsy with moderate dizziness. No other neurological sign or symptom was found. CT scan showed acute hydrocephalus secondary to a cystic lesion in the cerebellomedullary cistern. He was shunted first, with immediate relief of the symptoms. He was discharged home to complete antiparasitic drugs treatment. Eight months later, the patient complained of nausea and vomiting; a contrast MRI demonstrated the nonenhancing cystic lesion without scolex, extending to the fourth ventricle [
Figure 3:
(a) Preoperative axial view of a head computed tomography (CT) scan demonstrating an acute hydrocephalus (yellow arrow) secondary to neurocysticercosis (NCC) and (b) postoperative CT scan showing resolution (yellow arrow) after cerebrospinal fluid shunt. (c) Sagittal view of a T1-weighted magnetic resonance imaging (MRI) showing a racemose NCC in the cisterna magna, extending to the fourth ventricle (yellow arrows). (d) Axial fluid-attenuated inversion recovery MRI demonstrating an intraventricular cyst with surrounding ependymitis (e) intraoperative view of racemose NCC inside cisterna magna, before excision (f) intraoperative view after cyst resection, medulla oblongata (blue dot), left posteroinferior cerebellar artery (yellow arrow) with an atheromatous plaque and right cerebellar tonsil (black star).
CASE NO. 4
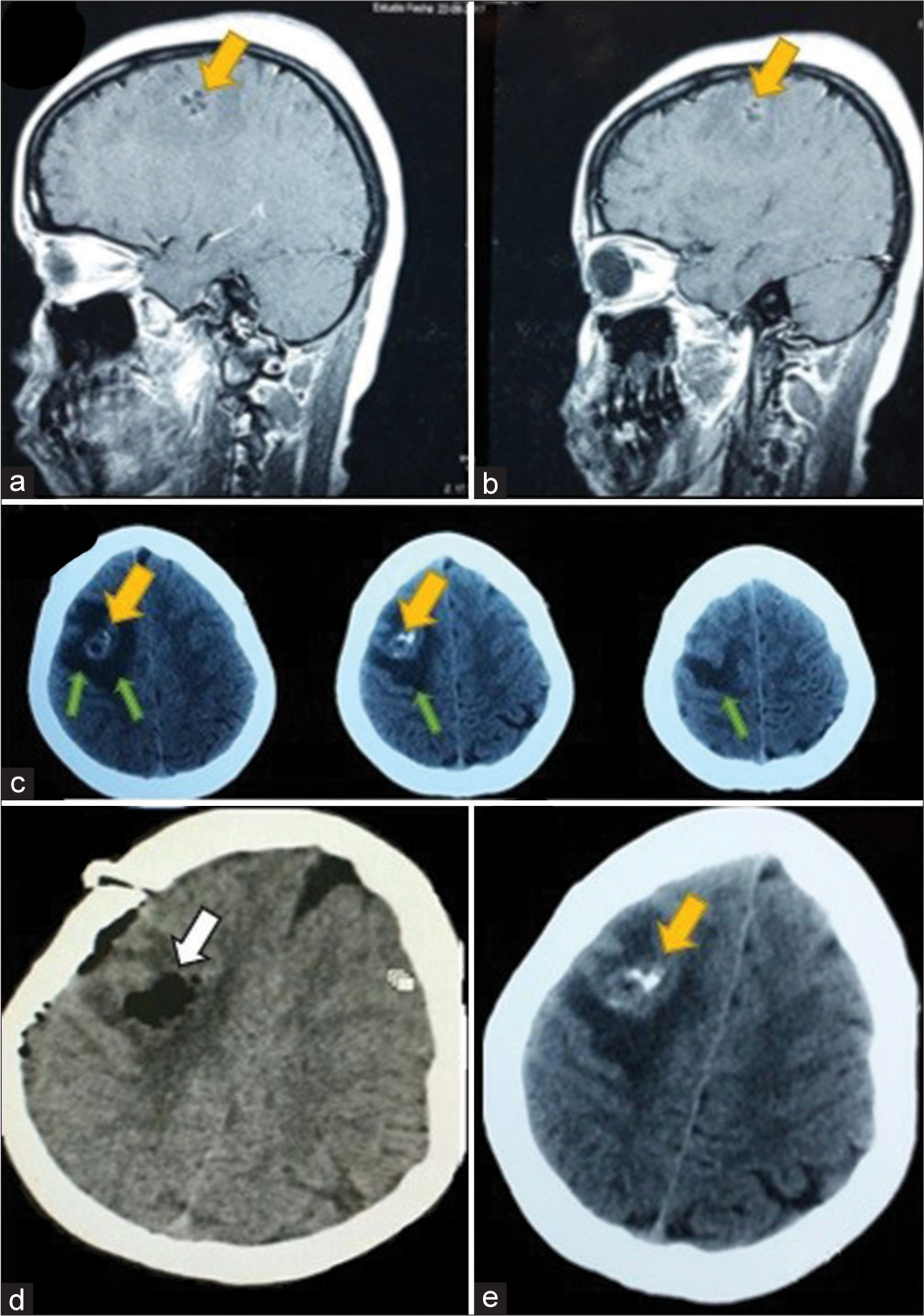
A 22-year-old female patient arrived at the emergency room presenting with generalized seizures, which had been preceded by a week of headaches. On examination using a head CT scan, an intracerebral cyst of middle size (2.3 × 2.1 × 3 cm) was identified in the right frontal lobe [
Figure 4:
(a and b) Sagittal view of a T1-contrasted sequence magnetic resonance imaging showing a middle size neurocysticercosis cyst (yellow arrow). (c) Axial view of a head CT scan showing the cyst (yellow arrow) and surrounding brain edema (green arrows). (d) Postoperative CT scan demonstrating the complete resection of the cyst and (e) preoperative axial view of a head CT scan showing the compressing cyst (yellow arrow).
Techniques
We performed a right frontal craniotomy, posterior middle frontal gyrus corticectomy, capsule coagulation, and complete removal using gentle traction. The lesion was solid, yellowish, and avascular.
RESULTS
The
DISCUSSION
The mainstay treatment of NCC involves symptomatic therapy using cysticidal drugs (praziquantel and albendazole) as first-line drugs.[
For practical purposes, these lesions can be classified according to the affected intracranial compartment (supratentorial, infratentorial, and spinal) or, more commonly: intraparenchymal/extraparenchymal (subarachnoid and intraventricular).[
Giant cyst variants (over 5 cm in its larger diameter)[
According to Sotelo and Marin, the mortality rate for hydrocephalus secondary to cysticercosis arachnoiditis was 50% (41 patients) in a period of 2 years after shunting.[
Microsurgical techniques are used for these pathologies, although it is not clear which of them are best suited.[
After drainage, easy handling using graspers to apply some traction helps develop a cleavage plane that can be continued using waterjet dissection (instilling warm saline between the cyst wall and the brain) with a small feeding tube or a small catheter attached to a syringe. Sometimes waterjet dissection can remove the whole cyst if there are no adhesions between the cyst wall and surrounding structures, similar to an Echinococcus hydatid cyst (Dowling-Orlando technique.[
Another practical microsurgical maneuver is a sharp dissection (transection of arachnoid webs and fibrous adhesions) when the cyst is in the subarachnoid space, especially in basal cisterns, that allows cyst traction without brain, nerve, or vascular (torn veins) damage.[
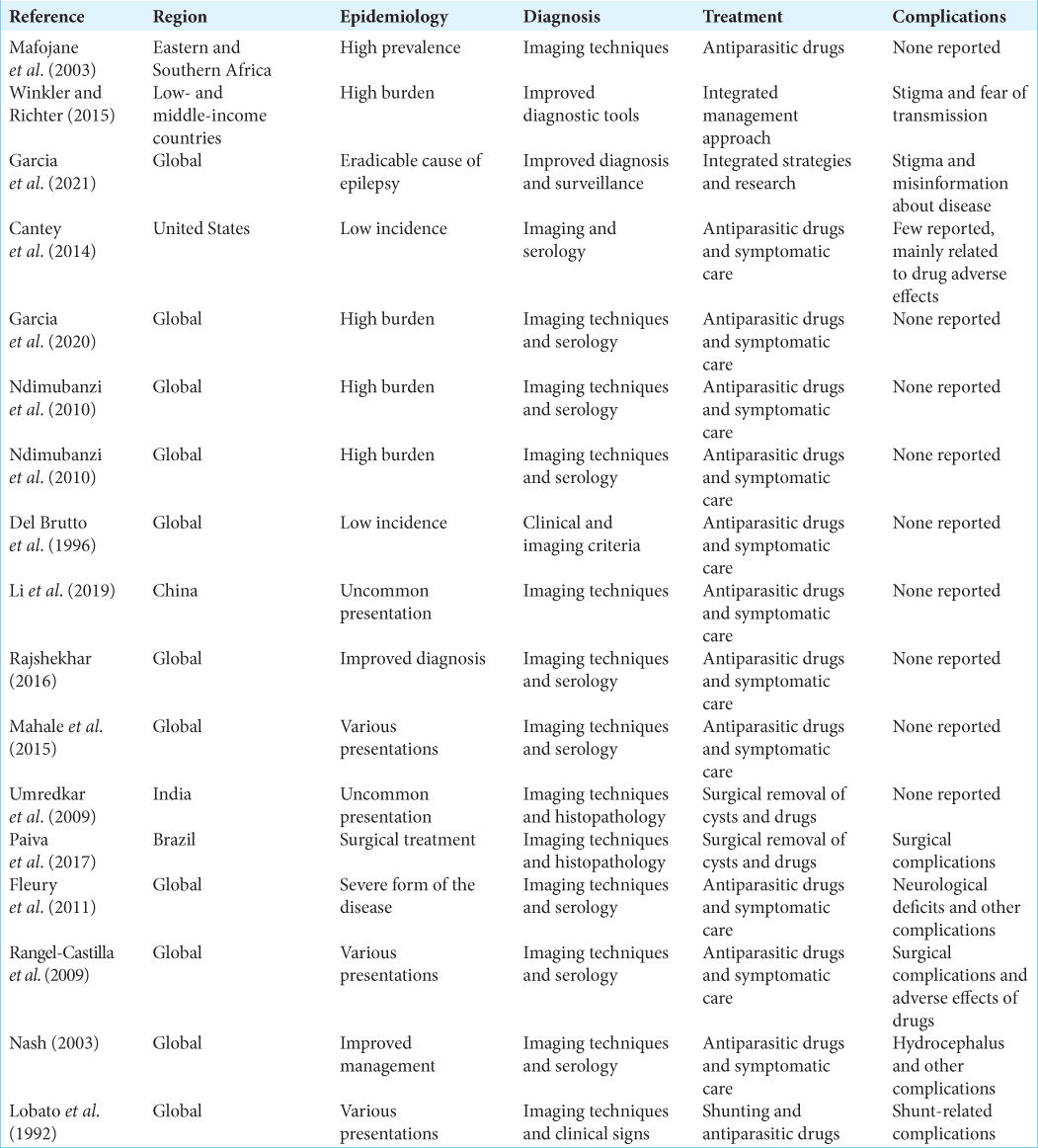
The results of the studies we reviewed suggest that surgical management can be an effective treatment option for giant neurocysticerci, with good or excellent outcomes achieved in a majority of cases. However, the success rates varied depending on the location of the cyst and the surgical approach used. In terms of intracranial location, the studies we reviewed included patients with giant neurocysticerci in various locations, including the intraventricular region, brainstem, basal ganglia, and supratentorial region. Overall, the studies suggested that surgical management can be successful regardless of the cyst location, as long as the surgical approach is tailored to the specific location. In terms of surgical approach, the studies we reviewed included craniotomy with cystectomy or aspiration, endoscopic surgery, and stereotactic aspiration. The choice of surgical approach depended on various factors, including cyst size and location, as well as surgeon’s preference and expertise. The studies suggested that both craniotomy and endoscopic surgery can be effective for removing giant neurocysticerci, but each approach has its own advantages and disadvantages. The studies also reported on complications associated with surgical management of giant neurocysticerci, including hemorrhage, infection, hydrocephalus, and neurological deficits. The incidence of complications varied across studies, but they were generally more common in patients who underwent craniotomy with cystectomy or aspiration compared to endoscopic surgery or stereotactic aspiration.
CONCLUSION
Surgical management is an important treatment option for giant neurocysticerci, especially in cases where medical management has failed or is contraindicated. The success rates and complication rates of different surgical approaches vary depending on the cyst location and surgeon expertise, but overall, surgical management can achieve good or excellent outcomes in a majority of cases. It is important for surgeons to carefully evaluate the patient’s clinical and radiographic features and to tailor the surgical approach to the specific cyst location and characteristics. In addition, close postoperative monitoring is necessary to detect and promptly treat any complications.
There is no formal data about NCC in the Dominican Republic so far in the literature. Giant NCC variant is a rare presentation, comprising 0.4–5% of all cases, and constitutes a real endemic problem in our country. Populated prospective studies with larger sample sizes are needed to better understand the clinical and surgical nuances of these rare entities and noncystic type lesions.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Balak N, Cavumirza C, Yildirim H, Ozdemir S, Kinay D. Microsurgery in the removal of a large cerebral hydatid cyst: Technical case report. Neurosurgery. 2006. 59: ONSE486
2. Bazan R, Filho PT, Luvizutto GJ, de Carvalho Nunes HR, Odashima NS, Dos Santos AC. Clinical symptoms, imaging features and cyst distribution in the cerebrospinal fluid compartments in patients with extraparenchymal neurocysticercosis. PLoS Negl Trop Dis. 2016. 10: e0005115
3. Cantey PT, Coyle CM, Sorvillo FJ, Wilkins PP, Starr MC, Nash TE. Neglected parasitic infections in the United States: Cysticercosis. Am J Trop Med Hyg. 2014. 90: 805-9
4. Colli BO, Carlotti CG, Assirati JA, Machado HR, Valença M, Amato MC. Surgical treatment of cerebral cysticercosis: Long-term results and prognostic factors. Neurosurg Focus. 2002. 12: e3
5. Del Brutto OH, Wadia NH, Dumas M, Cruz M, Tsang VC, Schantz PM. Proposal of diagnostic criteria for human cysticercosis and neurocysticercosis. J Neurol Sci. 1996. 142: 1-6
6. Fleury A, Carrillo-Mezo R, Flisser A, Sciutto E, Corona T. Subarachnoid basal neurocysticercosis: A focus on the most severe form of the disease. Expert Rev Anti Infect Ther. 2011. 9: 123-33
7. Garcia HH, Gonzalez AE, Gilman RH. Neurocysticercosis as an eradicable cause of epilepsy: A plan and actions are needed. JAMA Neurol. 2021. 78: 1045-6
8. Garcia HH, Gonzalez AE, Gilman RH. Taenia solium cysticercosis and its impact in neurological disease. Clin Microbiol Rev. 2020. 33: e00085-19
9. Li H, Sun J, Nan G. Nonspecific dizziness as an unusual presentation of neurocysticercosis: A case report. Medicine (Baltimore). 2019. 98: e16647
10. Lobato RD, Lamas E, Portillo JM, Roger R, Esparza J, Rivas JJ. Hydrocephalus in cerebral cysticercosis. Pathogenic and therapeutic considerations. J Neurosurg. 1981. 55: 786-93
11. Mafojane NA, Appleton CC, Krecek RC, Michael LM, Willingham AL. The current status of neurocysticercosis in Eastern and Southern Africa. Acta Trop. 2003. 87: 25-33
12. Mahale RR, Mehta A, Rangasetty S. Extraparenchymal (racemose) neurocysticercosis and its multitude manifestations: A comprehensive review. J Clin Neurol. 2015. 11: 203-11
13. Nash TE. Human case management and treatment of cysticercosis. Acta Trop. 2003. 87: 61-9
14. Ndimubanzi PC, Carabin H, Budke CM, Nguyen H, Qian YJ, Rainwater E. A systematic review of the frequency of neurocyticercosis with a focus on people with epilepsy. PLoS Negl Trop Dis. 2010. 4: e870
15. Paiva AL, Araujo JL, Ferraz VR, Lovato RM, Pedrozo CA, de Aguiar GB. Surgical treatment of neurocysticercosis. Retrospective cohort study and an illustrative case report. Sao Paulo Med J. 2017. 135: 146-9
16. Rajshekhar V. Neurocysticercosis: Diagnostic problems and current therapeutic strategies. Indian J Med Res. 2016. 144: 319-26
17. Rangel-Castilla L, Serpa JA, Gopinath SP, Graviss EA, DiazMarchan P, White AC. Contemporary neurosurgical approaches to neurocysticercosis. Am J Trop Med Hyg. 2009. 80: 373-8
18. Sharma BS, Sawarkar DP, Verma SK. Endoscopic management of fourth ventricle neurocysticercosis: Description of the new technique in a case series of 5 cases and review of the literature. World Neurosurg. 2019. 122: e647-54
19. Sotelo J, Marin C. Hydrocephalus secondary to cysticercotic arachnoiditis. A long-term follow-up review of 92 cases. J Neurosurg. 1987. 66: 686-9
20. Umredkar A, Singla N, Mohindra S, Bal A, Gupta SK. Giant intraparenchymal neurocysticercosis: Report of surgical aspects two cases. Neurol India. 2009. 57: 800-2
21. White AC, Coyle CM, Rajshekhar V, Singh G, Hauser WA, Mohanty A. Guidelines diagnosis and treatment of neurocysticercosis: 2017 clinical practice guidelines by the infectious diseases society of America (IDSA) and the American society of tropical medicine and hygiene (ASTMH). Am J Trop Med Hyg. 2018. 98: 945-66
22. Winkler A, Richter H, editors. Landscape Analysis: Management of Neurocysticercosis with an Emphasis on Low-and Middle-Income Countries. Geneva: World Health Organization; 2015. p.