- Department of Neurosurgery, Sana Clinic Offenbach, University of Frankfurt am Main academic Hospitals, Offenbach am Main, Mainz, Germany
- Department of Neurosurgery, Mainz University Medical Centre, Mainz, Germany,
- Department of Neurosurgery, Charing Cross Hospital, Imperial College Healthcare, London, United Kingdom,
- Department of Neurosurgery, Hamad General Hospital, Doha, Qatar,
- Department of Neurosurgery, Frankfurt am Main University Medical Centre, Frankfurt am Main, Mainz, Germany
- Institute of Anatomy, University Medical Center of the Johannes Gutenberg-University Mainz, Mainz, Germany.
Correspondence Address:
Lucas Serrano Sponton, Department of Neurosurgery, Sana Clinic Offenbach, University of Frankfurt am Main academic Hospitals, Offenbach am Main, Germany.
DOI:10.25259/SNI_107_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Lucas Serrano Sponton1, Eleftherios Archavlis2, Jens Conrad2, Amr Nimer3, Ali Ayyad4, Elke Januschek1, Daniel Jussen5, Marcus Czabanka5, Sven Schumann6, Sven Kantelhardt2. The classical supraorbital minicraniotomy to approach the areas of origin of anterior skull base meningiomas: Anatomical nuances influencing accessibility, operability, and frontal lobe retraction. 24-May-2024;15:168
How to cite this URL: Lucas Serrano Sponton1, Eleftherios Archavlis2, Jens Conrad2, Amr Nimer3, Ali Ayyad4, Elke Januschek1, Daniel Jussen5, Marcus Czabanka5, Sven Schumann6, Sven Kantelhardt2. The classical supraorbital minicraniotomy to approach the areas of origin of anterior skull base meningiomas: Anatomical nuances influencing accessibility, operability, and frontal lobe retraction. 24-May-2024;15:168. Available from: https://surgicalneurologyint.com/surgicalint-articles/12911/
Abstract
Background: The classical supraorbital minicraniotomy (cSOM) constitutes a minimally invasive alternative for the resection of anterior skull base meningiomas (ASBM). Surgical success depends strongly on optimal patient selection and surgery planning, for which a careful assessment of tumor characteristics, approach trajectory, and bony anterior skull base anatomy is required. Still, morphometrical studies searching for relevant anatomical factors with surgical relevance when intending a cSOM for ASBM resection are lacking.
Methods: Bilateral cSOM was done in five formaldehyde-fixed heads toward the areas of origin of ASBM. Morphometrical data with potential relevant surgical implications were analyzed.
Results: The more tangential position of the cSOM with respect to the olfactory groove (OG) led to a reduction in surgical freedom (SF) in this area compared to others (P P P P P
Conclusion: Although clinical validation is still needed, the present anatomical data suggest that assessing minicraniotomy’s position/extension, OG depth, the sphenoid’s slope, and distance to ACP-tip might be of particular relevance to predict FLR, maneuverability, and accessibility when considering the cSOM for ASBM resection, thus helping surgeons optimize patient selection and surgical strategy.
Keywords: Anatomy, Classical supraorbital approach, Meningioma, Skull base
INTRODUCTION
The use of classical supraorbital minicraniotomy (cSOM) constitutes a minimally invasive transcranial alternative to standard pterional, frontolateral, and sub-frontal craniotomies for the treatment of pathological processes within the rostral skull and brain, including the resection of anterior skull base meningiomas (ASBM).[
When facing the questions of whether a cSOM may be effective and safe for ASBM resection and which specific technical considerations may be required for surgery, three main issues are to be assessed preoperatively. First, the evaluation of tumor characteristics, such as size, growing pattern, and relationship (possible adherence) to main neurovascular structures, is key for predicting the feasibility of debulking and dissecting the tumor dome using a cSOM, the amount of brain retraction required for this task, potential risks of the neurovascular lesion, and the possibility to solve them intraoperatively through the small craniotomy. Second, assessing the spatial relationship between the craniotomy’s position and the area where the tumor is located is particularly important in keyhole approaches (as is the case of the cSOM) to foresee limitations for tumor resection or intraoperative complication management driven by an eventual too tangential approach trajectory or the presence of interposing structures along the narrower access route. Last but not least, evaluating the individual bony skull base anatomy is fundamental to predict the accessibility through the minicraniotomy to the most basal portions of the tumor and its matrix (especially in deep areas such as the olfactory groove [OG]) and the amount of brain retraction required to work in this area. A correct visualization and instrument maneuverability along the matrix are important to avoid leaving potentially removable tumor remanent, spare neurovascular damage (especially next to the anterior clinoid or tuberculum sellae [TS]), as well as enable managing eventual complications (such as bleedings coming from this often highly vascularized area) or unintended dural openings, associated with cerebrospinal-fluid leaks. On the other hand, visualization and maneuverability to get access to deep regions of the anterior skull base should be accomplished, minimizing brain retraction, which may otherwise increase the risk of cerebral contusion, swelling, and postoperative neurological deterioration.
To date, most of the recommendations for surgery performance and patient selection for ASBM resection through a cSOM are based on analyzing tumor-specific features, such as size, growing pattern, and relationship to neurovascular structures.[
MATERIALS AND METHODS
Five adult human head specimens, conserved through arterial perfusion with a formaldehyde solution of 40 g/L and subsequent formaldehyde immersion within humidity chambers, were subjected to bilateral cSOM, completing a total of n = 10 supraorbital approaches. Dissections were performed following strict hygienic and ethical standards.
The target regions were defined as those where ASBM usually arise, namely, the OG, planum sphenoidale (PS), TS, and anterior clinoid process (ACP). Heads were mounted on a 19 × 19 cm quadrangular holder and fixed at 4 points with adjustable pins. The surgical technique resembled the one used in the clinical series for the resection of ASBM.[
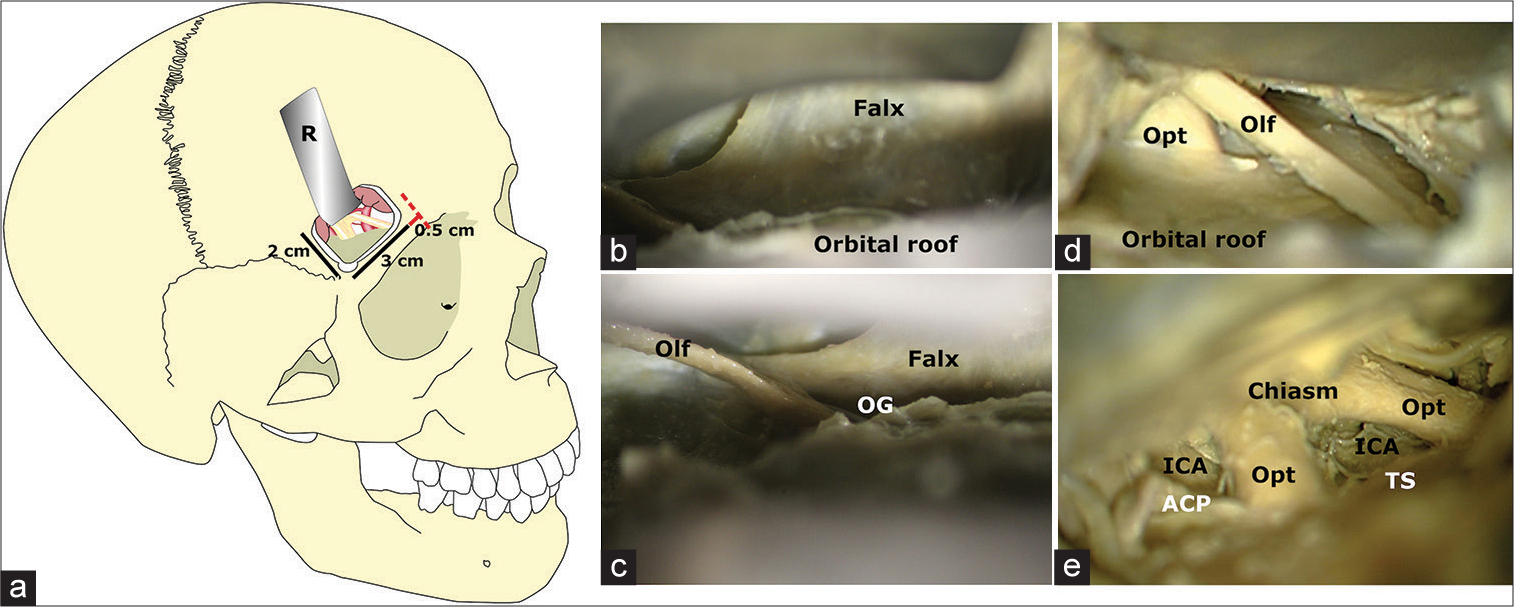
Figure 1:
Craniotomy landmarks and anatomical dissections. (a) The medial boundary of each classical supraorbital minicraniotomy was located 0.5 cm lateral (red line) to the supraorbital notch (red dotted line), from which all craniotomies extended 3 cm laterally and 2 cm cranially. (b-e) After placing a single brain retractor (R) below the frontal lobe, a stepwise anterior skull base dissection was carried on. A careful exposure of the anterior fossa and related relevant neurovascular structures was accomplished before morphometrical assessment as depicted from more anterior to posterior. ACP: Anterior clinoid process; ICA: Internal carotid artery; OG: Olfactory groove; Olf: Olfactory nerve, Opt: Optic nerve, TS: Tuberculum sellae.
Assessed morphometrical parameters included the distances and approach angles to all the structures mentioned above. Parameters were recorded in relation to the craniotomy’s midpoint (situated at the caudal rim of the craniotomy exactly between the lateral and medial margin) at the outer skull [
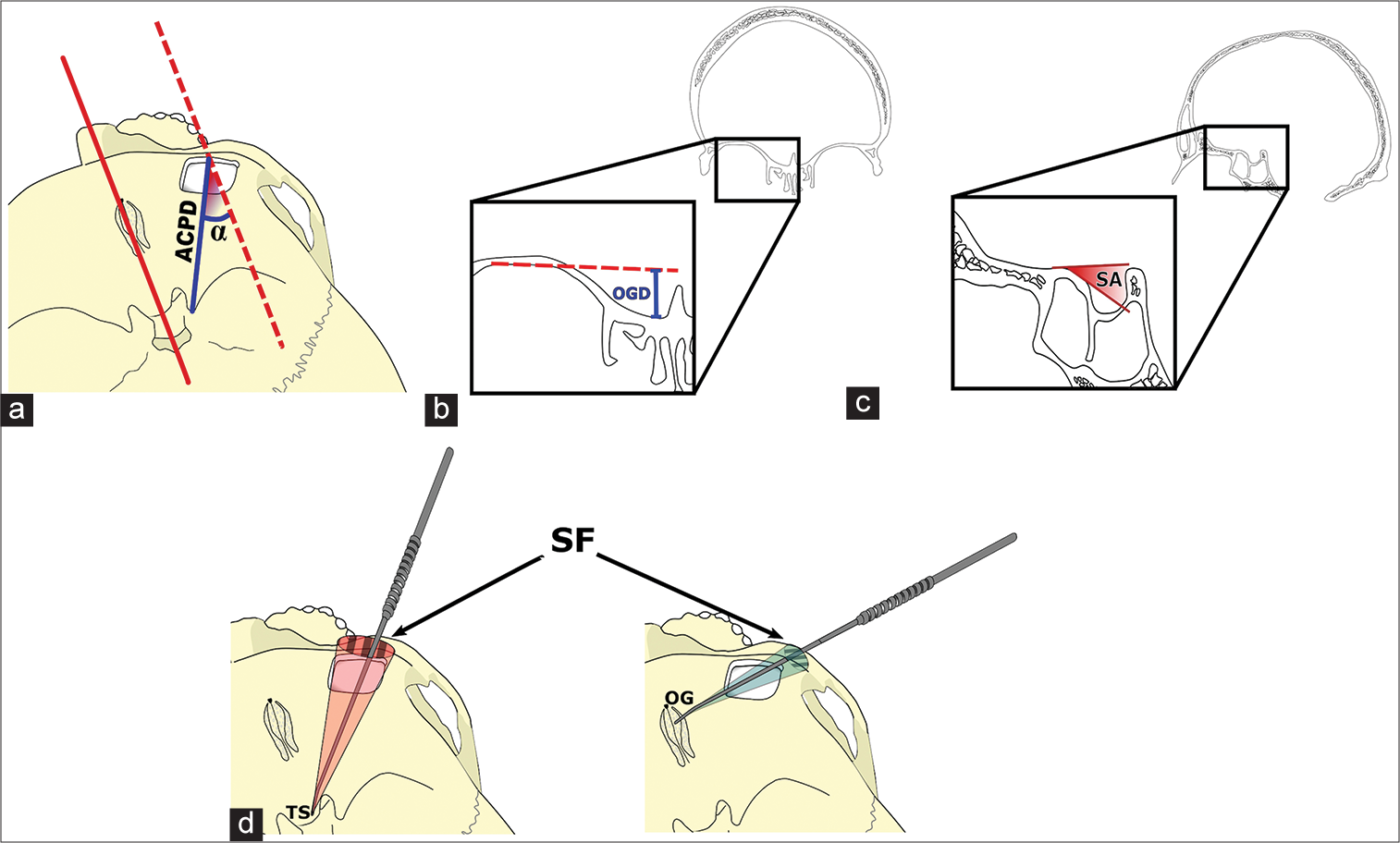
Figure 2:
Schematic representation of morphometrical measures. (a) The distances to all assessed target structures, in this example, the distance to the anterior clinoid process (ACPD, blue line) ACPD (blue line), were measured from the craniotomy’s midpoint, whereas the approach angles (a) were taken in relationship to a reference axis (red dotted line) running parallel to the midline. (b) The olfactory groove’s depth (OGD) was measured in the coronal plane with respect to the orbital roof after smoothing its inner bony layer (red dotted line). (c) The sphenoid angle (SA) was measured as the slope descending toward the sella with respect to the planum sphenoidale’s axis. (d) Surgical freedom (SF) (striped pattern) was assessed as the area covered 3 cm above the craniotomy by a free-moving dissector with its tip fixed on each of the target landmarks. OG: Olfactory groove, TS: Tuberculum sellae.
Statistical analysis
For statistical analysis, Statistical Package for the Social Sciences Statistics 19.0 v (IBM Corp., Armonk, NY, USA) and GraphPad Prism 9.0.0 (GraphPad Software Inc., San Diego, CA, USA) were used. All data were subjected to a descriptive data analysis. Shapiro–Wilk goodness of fit tests were done to determine the parametric or non-parametric distribution of each variable. Since a normal distribution could be assumed for all variables, central tendency measures are expressed as the arithmetic mean and its dispersion as the standard deviation.
Student-t-tests and analysis of variance (ANOVA) were used for comparing two and three or more means, respectively. The effect size for significant ANOVA P-values was expressed as partial eta-square (ηp2). Correlation analyses were performed calculating Pearson’s r correlation coefficient. The respective level of significance is identified as follows: ****P < 0.0001, ***P < 0.001, **P < 0.01, and *P < 0.05.
RESULTS
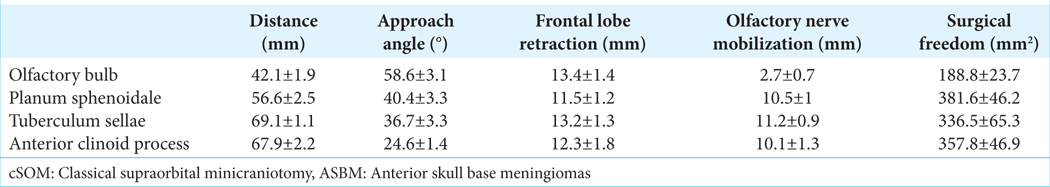
Distances and approach angles to the OG, PS, TS, and the tip of the ACP (ACPD) are depicted in
The approach angle increased from lateral toward medial and anteromedial structures, reaching a maximum of 59 ± 3° for the OG. The most distant structures targeted through the cSOA, that is, the tip of the ACP and the TS, were found at distances of 67.9 ± 2.2 and 69.1 ± 1.1 mm, respectively. OGD averaged 12.5 ± 1.9 mm and the SA 53.6 ± 7°. Mobilization of the olfactory nerve was specifically required when approaching the PS, TS, and ACP. A significantly lower olfactory nerve mobilization (mostly passive due to frontal lobe elevation) was required when approaching the olfactory bulb, as demonstrated in univariate ANOVA (F[4,50] = 178,621, P < 0001, ηp2 = 0.941) and T-tests (all P < 0.0001), [
Figure 3:
(a) Olfactory nerve mobilization, (b) surgical freedom, and (c) frontal lobe retraction required to reach the target anatomical landmarks assessed on the anterior skull base. Bars represent means ± 2 standard error. OG: Olfactory groove, PS: Planum sphenoidale, TS: Tuberculum sellae, ACP: Anterior clinoid process. * p<0.05, **** p<0.0001
SF on the OG, averaging 188.8 ± 23.6 mm2, was significantly lower when compared to the other assessed regions, as demonstrated in univariate ANOVA (F[3,36] = 33, P < 0.0001, ηp2 = 0.726) and T-tests (all P < 0.0001); [
Figure 4:
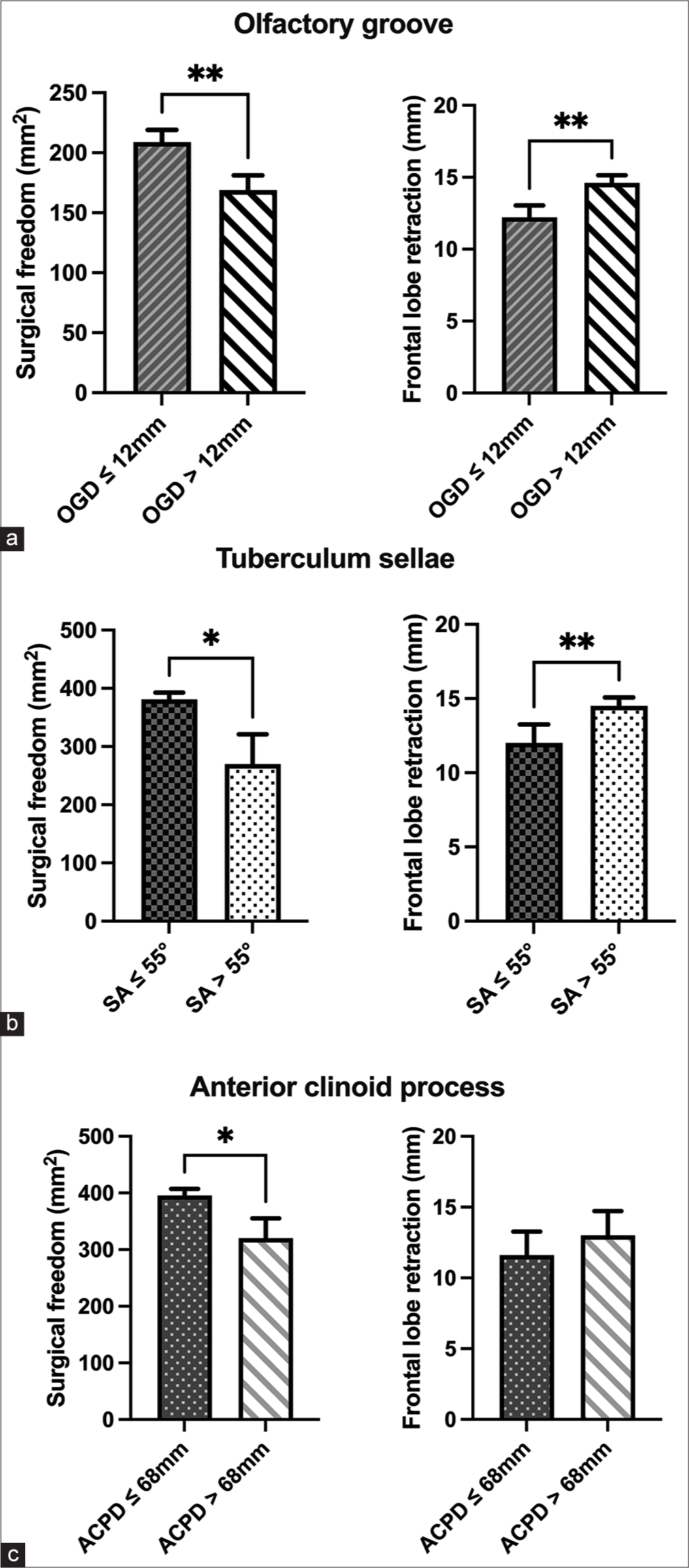
Analysis of surgical freedom (left) and frontal lobe retraction (right) required when approaching the (a) olfactory groove, (b) tuberculum sellae, and anterior clinoid process (c) according to olfactory groove’s depth (OGD), sphenoid angle (SA), and distance to the anterior clinoid process tip (ACPD), respectively. Bars represent means ± 2 standard error. *p<0.05, ** p<0.01.
In addition, a strong negative correlation was found between SA amplitude and the SF along the TS (r = −0.787, P < 0.01). When specimens were divided according to SA, t-tests demonstrated a significant SF reduction along the TS (P < 0.05) for those with an SA > 55° [
The FLR required to achieve maximal exposure of the analyzed structures was maximum for the OG, followed by the TS, with average values of 13.4 ± 1.4 and 13.2 ± 1.3 mm, respectively. In contrast, FLR was minimal to the PS, averaging 11.5 ± 1.2 mm [
DISCUSSION
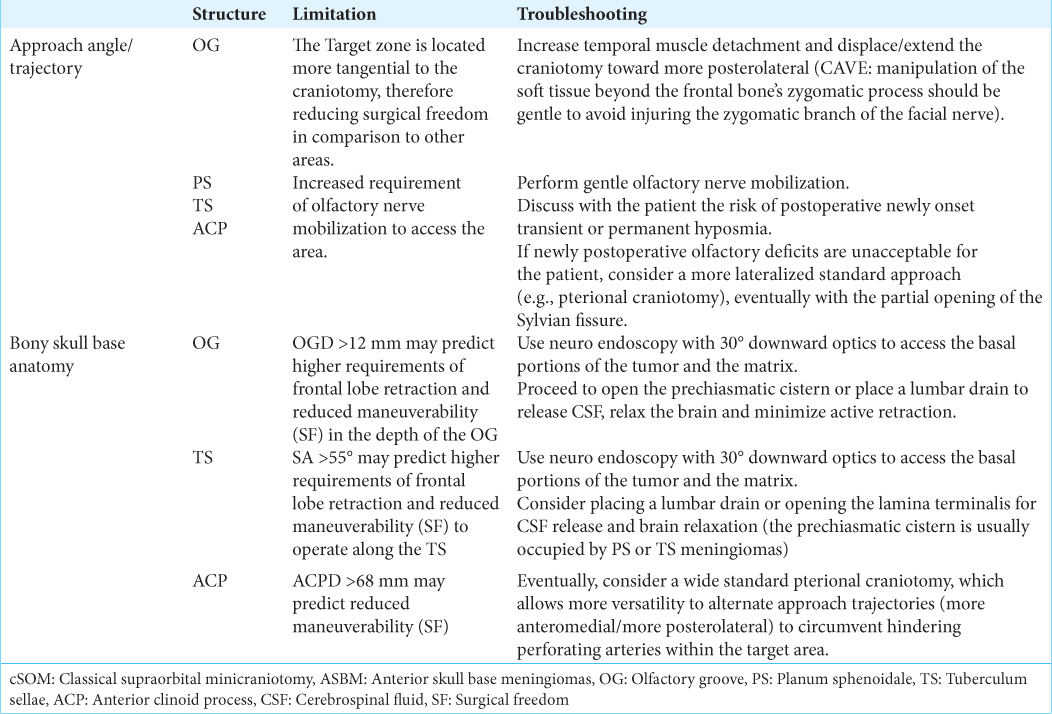
This study was designed to analyze factors regarding approach angles and trajectories, as well as specific bony skull base features, which have not been thoroughly addressed in the published literature but may have relevant implications for patient selection and surgical performance when considering a cSOM for the specific case of ASBM resection. Our results demonstrate that given the position of the cSOM and the resulting approach trajectories to the areas where ASBM arise, the surgical maneuverability (represented by the SF) is significantly more limited along the OG in comparison to other more straightforward-located structures, such as the TS and PS. Approaching the TS, ACP, and PS requires, however, a significantly higher amount of olfactory nerve mobilization. When applying a cSOM, the steeper trajectory toward the OG significantly increases the requirement of brain retraction in the depth of this area. The most relevant findings in our work were that OGD and the increasing slope toward the sella (SA) represent important predicting factors for SF and FLR along the OG and the TS, whereas an increasing ACPD may predict reduced surgical maneuverability within the ACP. We found no specific bony anatomical parameters influencing the SF or FLR required to access the PS.
Prior morphometrical data focusing on the working space around vascular elements, such as the middle cerebral artery (MCA) bifurcation, the most distal point of the ipsi- or contralateral posterior cerebral artery and the contralateral MCA, demonstrated that the cSOM might eventually provide similar surgical working space in depth as the pterional and orbitozygomatic approach if patients are carefully selected.[
Another observation of our study was that after applying the cSOM, the trajectory to the target structures behind the olfactory bulb was partially obstructed by the olfactory nerve. Consequently, olfactory nerve mobilization was analyzed and the results demonstrated a significant amount of mobilization needed for accessing the PS, TS, and ACP. Olfactory nerve dysfunction constitutes a frequently reported side effect in ASBM surgery.[
The present results also endorse the relevance of specific bony skull base features for predicting the needs of FLR and surgical maneuverability along the areas where the matrix end most basal portions of ASBM are located. First, we observed that the deeper the OG, the greater the FLR required and the lower the SF to operate in this area, suggesting that preoperative evaluation of OGD should be mandatory when approaching the OG through a cSOM. Again, although the presence of a deep OG does not necessarily implicate the contraindication of a supraorbital approach, it may have direct consequences on the surgical technique. In cases of meningiomas arising from an OG deeper than 12 mm, the inclusion in the surgical armamentarium of neuro endoscopy with 30°-optics, as well as angled microinstruments will be particularly useful for safe tumor resection along the deep-located matrix, increasing SF, and minimizing brain retraction. Since performing skilled surgical maneuvers guided by angled endoscopic views may represent a difficult task for the not-experienced neurosurgeon, considering this factor before surgery may be important to prevent unexpected difficulties during deeper tumor detachment or deal with eventual intraoperative complications (e.g., in case of needing to seal a cerebrospinal-fluid fistula along the cribriform plate or cauterizing bleedings along the tumor matrix coming from ethmoidal artery branches). In addition, the presence of OG deeper than 12 mm indicates that, independently of tumor size, surgical measures to maximize brain relaxation (such as opening the prechiasmatic cistern or placing a lumbar drain before surgery) are strongly encouraged to reduce the forces applied with the retractors. Similarly, a steeper sphenoidal slope (i.e., SA) was associated with a reduced SF along the TS, a fact related to a higher limitation to move the instruments along a sinking surface below the approach’s plane and higher FLR required. Based on these data, we suggest that preoperative detection of a SA >55° should alert the surgeon about an increasing difficulty in operating on the basal portions of the tumor in this area, thus highlighting the need to use endoscopic assistance with downward 30°-optics and curved microinstruments to overcome possible limitations. Since the prechiasmatic cistern is usually occupied by TS meningiomas, the presence of a SA >55° could be considered a practical reference for surgeons to consider cerebrospinal fluid release through a lamina-terminalis opening or preoperative placement of lumbar drainage, achieving in this way maximal brain relaxation and reducing the pressure applied by the retractor over the frontal lobe.
In the case of the ACP, the more frequent interposition of perforating arteries around the optic nerve and supra clinoid carotid artery acted as the main factor linked to SF reduction in specimens with longer ACPD. This fact may also explain why only SF and not FLR were influenced by ACPD since not the approach’s slope, but rather obstructing vascular elements increased the difficulties to access the area. ACPD could be eventually used to determine which patients may be candidates for a cSOM or whether a more lateral trajectory applying wider craniotomies (e.g., a front lateral craniotomy or a pterional approach with Sylvian-fissure dissection) should be preferred to circumvent hindering perforating arteries within the target area. On the other hand, since perforating arteries might be displaced toward the front, behind or above an ACP meningioma, it turns questionable in this case whether the sole calculation of ACPD may be useful to predict SF in cases of tumor or masses affecting the ACP directly. ACPD could probably play a clearer role in predicting surgical maneuverability in cases where the anatomy along the superior clinoid’s surface is not distorted and the pathology is located posterior to the ACP (such, for example in posterior communicating aneurysms).[
In summary, the anatomical data here presented provide new insights for patient selection and surgical performance when considering a cSOM for the resection of ASBM, going beyond the recommendations based basically on tumor features and endorsing the importance of considering additional factors associated with the approach’s trajectory and bony anterior skull base anatomy [
CONCLUSION
The cSOM enables a more esthetical and minimally invasive approach to ASBM, but the key to success relies on a very careful patient selection surgical planning and technical performance. Available retrospective surgical data provide useful recommendations for surgeons based mostly on tumor-related features. Our present results demonstrate that specific anatomical characteristics related to the approach trajectory and bony skull base are also relevant in the preoperative assessment when considering a cSOM to approach the areas where ASBM usually arise. Particularly, a more posterolateral craniotomy extension may be useful to overcome the more reduced surgical maneuverability along the OG. Furthermore, assessing OGD, SA, and ACPD seems to be of practical relevance for decision-making destined to avoid excessive FLR and maximize operability in the basal portions of OG, TS, and ACP meningiomas. Future prospective surgical trials should be encouraged to determine further the role played by all anatomical and tumor associated factors for the effective and safe performance of ASBM resections through the cSOM.
Availability of data and material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Authors’ contributions
Lucas Serrano Sponton, Eleftherios Archavlis, and Sven Kantelhardt conceptualized this study. Lucas Serrano Sponton performed anatomical dissections and measurements, analyzed the data, and wrote the original manuscript. Eleftherios Archavlis, Jens Conrad, Amer Nimer, Ali Ayyad, Elke Januschek, Daniel Jussen, and Marcus Czabanka edited the original manuscript. Sven Schumann administrated anatomical resources. Sven Schumann and SK administrated the project. All authors reviewed the manuscript critically and approved for publication of the content.
Ethical approval
The research/study was approved by the Institutional Review Board at Rhineland Palatinate Ethical Committee, number 2022-16595, dated October 14, 2022.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
The authors thank very much Mr. Thomas Bauer for his assistance with drawings for Figures 1 and 2, which greatly helped to improve the message of our manuscript. We sincerely thank those who donated their bodies to science so that anatomical research could be performed. Results from such research may potentially increase mankind’s overall knowledge, improving the patient care. Therefore, these donors and their families deserve our highest gratitude.
References
1. Blomqvist EH, Brämerson A, Stjärne P, Nordin S. Consequences of olfactory loss and adopted coping strategies. Rhinology. 2004. 42: 189-94
2. Ciurea AV, Iencean SM, Rizea RE, Brehar FM. Olfactory groove meningiomas. Neurosurg Rev. 2012. 35: 195-202
3. Dedeciusova M, Svoboda N, Benes V, Astl J, Netuka D. Olfaction in olfactory groove meningiomas. J Neurol Surg A Cent Eur Neurosurg. 2020. 81: 310-7
4. Deems DA, Doty RL, Settle RG, Moore-Gillon V, Shaman P, Mester AF. Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch Otolaryngol Head Neck Surg. 1991. 117: 519-28
5. Fatemi N, Dusick JR, de Paiva Neto MA, Malkasian D, Kelly DF. Endonasal versus supraorbital keyhole removal of craniopharyngiomas and tuberculum sellae meningiomas. Neurosurgery. 2009. 64: 269-87
6. Fernandes YB, Maitrot D, Kehrli P, de Tella Jr, Ramina R, Borges G. Supraorbital eyebrow approach to skull base lesions. Arq Neuropsiquiatr. 2002. 60: 246-50
7. Figueiredo EG, Deshmukh V, Nakaji P, Deshmukh P, Crusius MU, Crawford N. An anatomical evaluation of the mini-supraorbital approach and comparison with standard craniotomies. Neurosurgery. 2006. 59: ONS212-20 discussion ONS220
8. González-Darder JM, Quilis-Quesada V, Talamantes-Escribá F, Botella-Maciá L, Verdú-López F. Microsurgical relations between internal carotid artery-posterior communicating artery (ICAPComA) segment aneurysms and skull base: An anatomoclinical study. J Neurol Surg B Skull Base. 2012. 73: 337-41
9. Hummel T, Nesztler C, Kallert S, Kobal G, Bende M, Nordin S. Gustatory sensitivity in patients with anosmia. Chem Senses. 2001. 26: 1118
10. Khan DZ, Muskens IS, Mekary RA, Zamanipoor Najafabadi AH, Helmy AE, Reisch R. The endoscope-assisted supraorbital “keyhole” approach for anterior skull base meningiomas: An updated meta-analysis. Acta Neurochir (Wien). 2021. 163: 661-76
11. Kobayashi M, Costanzo RM. Olfactory nerve recovery following mild and severe injury and the efficacy of dexamethasone treatment. Chem Senses. 2009. 34: 573-80
12. Komotar RJ, Starke RM, Raper DM, Anand VK, Schwartz TH. Endoscopic skull base surgery: A comprehensive comparison with open transcranial approaches. Br J Neurosurg. 2012. 26: 637-48
13. Park SK, Shin YS, Lim YC, Chung J. Preoperative predictive value of the necessity for anterior clinoidectomy in posterior communicating artery aneurysm clipping. Neurosurgery. 2009. 65: 281-5 discussion 285-6
14. Reisch R, Marcus HJ, Hugelshofer M, Koechlin NO, Stadie A, Kockro RA. Patients’ cosmetic satisfaction, pain, and functional outcomes after supraorbital craniotomy through an eyebrow incision. J Neurosurg. 2014. 121: 730-4
15. Reisch R, Perneczky A. Ten-year experience with the supraorbital subfrontal approach through an eyebrow skin incision. Neurosurgery. 2005. 57: 242-55
16. Reisch R, Perneczky A, Filippi R. Surgical technique of the supraorbital key-hole craniotomy. Surg Neurol. 2003. 59: 223-7
17. Reisch R, Stadie A, Kockro RA, Hopf N. The keyhole concept in neurosurgery. World Neurosurg. 2013. 79: S17.e9-13
18. Santos DV, Reiter ER, DiNardo LJ, Costanzo RM. Hazardous events associated with impaired olfactory function. Arch Otolaryngol Head Neck Surg. 2004. 130: 317-9
19. Serrano Sponton L, Alhoobi M, Archavlis E, Shaaban AT, Dumour E, Nimer A. Endoscopic-assisted paramedian supracerebellar infratentorial approach to the posterior portion of the third ventricle. Anatomical study and surgical cases. J Neurosurg Sci. 2022. p.
20. Sponton LS, Archavlis E, Conrad J, Nimer A, Ayyad A, Januschek E. Variants of the anterior subtemporal approach to the Gasserian ganglion and related structures: An anatomical study with relevant implications for keyhole surgery. World Neurosurg. 2023. 176: e587-97
21. Sponton LS, Oehlschlaegel F, Nimer A, Schwandt E, Glaser M, Archavlis E. The endoscopic-assisted supraorbital approach for resection of anterior skull base meningiomas: A large single-center retrospective surgical study. J Neurol Surg B Skull Base. 2023. 84: 349-60
22. Sponton LS, Shaaban AT, Archavlis E, Alhoobi M, Nimer A, Conrad J. Two-stage endoscopic assisted approach for large pineal region and falcotentorial meningioma: first stage paramedian supracerebellar infratentorial approach, second stage interhemispheric occipital transtentorial approach: Surgical cases and anatomical study. Neurosurg Rev. 2022. 45: 1759-72
23. Symon L, Rosenstein J. Surgical management of suprasellar meningioma: Part 1: The influence of tumor size, duration of symptoms, and microsurgery on surgical outcome in 101 consecutive cases. J Neurosurg. 1984. 61: 633-41
24. Telera S, Carapella CM, Caroli F, Crispo F, Cristalli G, Raus L. Supraorbital keyhole approach for removal of midline anterior cranial fossa meningiomas: A series of 20 consecutive cases. Neurosurg Rev. 2012. 35: 67-83
25. Welge-Luessen A, Temmel A, Quint C, Moll B, Wolf S, Hummel T. Olfactory function in patients with olfactory groove meningioma. J Neurol Neurosurg Psychiatry. 2001. 70: 218-21
26. Zhang M, Wang L, Zhang W, Qi W, Wang R, Han XD. The supraorbital keyhole approach with eyebrow incisions for treating lesions in the anterior fossa and sellar region. Chin Med J (Engl). 2004. 117: 323-6